November 8, 2017 — San Diego,CA Intrauterine devices (IUD) have become an increasingly popular choice of contraception among women looking to postpone having children. The use of long-acting reversible contraceptives increased nearly five-fold in the past decade, from 1.5 percent in 2002 to 7.2 percent in 2013, according to the Centers for Disease Control and Prevention..
Women often opt for an IUD over traditional forms of contraception like the pill because it offers prolonged contraception without a daily regimen. IUDs can prevent pregnancy for three, five, even 10 years before they lose their effectiveness. This may be one of the reasons they have become so popular in recent years.
Like most forms of contraception, IUDs carry inherent risks and these 
Mirena IUD has come under increased criticism for the number of complications being reported by women: As of June 2017 there were close to two-hundred lawsuits filed against Bayer Pharmaceuticals alleging Mirena caused them to suffer from pseudotumor cerebri (also known as intracranial hypertension). An article written and published in 1995 by the New England Journal of Medicine showed a link between levonorgestrel with intracranial hypertension. It appears that there may be many more women joining this lawsuit.
From the study: “Our patients were a 16-year-old white girl and a 19-year-old Hispanic woman in whom disk edema occurred four to five months after the implantation of levonorgestrel. Neither was obese, had prior medical problems, or used other medications that have been associated with intracranial hypertension. Both had normal results on neuroimaging studies (magnetic resonance imaging or computed tomography), and both had lumbar-puncture opening pressures that exceeded 550 mm of water with normal chemical and cellular analysis. Presenting symptoms consisted of transient visual obscurations, severe headaches, or emesis. Ocular findings included visual acuity in the range of 20/20 to 20/25 and florid bilateral papilledema with enlarged blind spots. Intracranial hypertension resolved in the 19-year-old patient; she remains free of symptoms after 28 months of follow-up. The 16-year-old patient has had three recurrences over a period of 18 months, each of which was controlled with acetazolamide.”
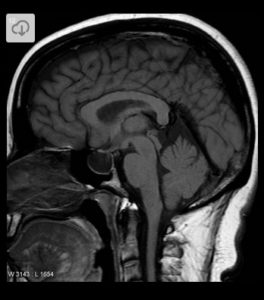
Mirena IUD may be linked to pseudotumor cerebri.
Pseudotumor cerebri (intracranial hypertension) is a condition that mimics a brain tumor, but it is not a tumor. Symptoms often mimic a brain tumor, but there is no tumor is present.
Treatment options for pseudotumor cerebri
Women who suffer from pseudotumor cerebri can have vision problems, and many times doctors will provide different options for preserving the optic nerve function.
Optic Nerve Sheath Fenestration: This surgical option is best for those experiencing vision loss and papilledema (pressure on the brain that causes the optic nerve to swell).
It involves cutting small slits or square patches in the dura that surrounds the optic nerve behind the globe. By doing so this helps drain cerebrospinal fluid (CSF) into the orbital fat layer where it is then absorbed.
This technique has been shown to reduce headaches in some patients and helps with reversing nerve edema. Other techniques include: optic nerve sheath decompression, ventriculoperitoneal and lumboperitoneal shunting, and intracranial venous sinus stenting.
What is the Mirena IUD?
Mirena is manufactured by Bayer Healthcare and has quickly become one of the most popular choices of IUDs among women in the United States. The device was approved by the Food and Drug Administration (FDA) in December 2000 when it was being manufactured by Berlex Laboratories Inc. Berlex was acquired by Bayer six years later.
Mirena is a flexible plastic device inserted into the uterus by a physician. Mirena is not surgically inserted, and according to the Bayer website, it usually takes only a few minutes for insertion to be completed. Some women may experience pain during insertion, but the severity of the pain varies from woman to woman.
Mirena works by releasing small amounts of the hormone progestin into the uterus which thickens cervical mucus and prevents sperm from passing into the uterus. If placed correctly, Mirena is usually over 99 percent effective in preventing pregnancy and lasts up to five years. After five years, women can choose to get another IUD inserted or have children if they are ready; IUDs typically do not impact future fertility.
Sound too good to be true? For some women, it is. Mirena can cause serious, potentially life-threatening side effects.
Mirena can migrate, perforate organs
When women choose to have an IUD inserted, they are accepting certain risks. One of those risks is perforation of the uterus or nearby organs.
In both clinical trials and real-world practice, Mirena has been shown to perforate (pierce) the uterus or cervix. In some cases, Mirena can perforate the uterus and migrate into organs in the pelvic or abdominal cavity, including the bladder. In rare cases, the device can perforate the rectum.
Perforation is a potentially serious complication and can cause scarring, infection and damage to other organs. The risk of perforation is about one in 1,000 users.
Studies show that perforation is usually the result of poor placement of the device within the uterus. If the device migrates, it may not protect a woman against unwanted pregnancy. A perforated device must be removed and surgery may be required to do so.
Breastfeeding women have a higher risk of perforation because their uterine walls are softer. The Mirena label was updated in 2013 to indicate this risk.
Other complications associated with Mirena
Pseudotumor cerebri (intracranial hypertension).
Expulsion
Even if the device doesn’t perforate the uterine wall, it can still be expelled from the correct position and increase the risk for unwanted pregnancy. It is possible for the device to slip slightly from the correct position, called partial expulsion, or it can slip completely out of place, called complete expulsion. A device that is expelled must be removed.
Sepsis
Sepsis is a potentially life-threatening complication that can occur within the first few days of insertion. Sepsis occurs when the chemicals released by the body into the blood stream to fight an infection trigger inflammation, which can lead to tissue damage, organ failure and death.
Ectopic pregnancy
The risk of pregnancy while the Mirena device is inserted is about one per 1,000 users, but half of all pregnancies that do occur are ectopic. Ectopic pregnancies occur when the egg implants itself in the fallopian tube instead of the uterus. Ectopic pregnancies require emergency treatment as they can cause internal bleeding, infertility and even death.
Women file lawsuits against Bayer
Women who were injured by the Mirena IUD are filing lawsuits against Bayer Healthcare saying the company failed to warn them of potential risks of the device.
If you or a loved one was injured or diagnosed with pseudotumor cerebri (intracranial hypertension) from the Mirena IUD, you may be entitled to cash compensation payouts from claims and settlements, but you need to act now as cases are currently pending in U.S. federal court.
If you or someone you love has suffered as a result of trusting this form of birth control, you need to make sure that you obtain the help of Mirena pseudotumor cerebri lawsuit lawyers who have been holding corporations accountable for the harm they have done for decades. Contact the Hood National Law Group, at 1-800-214-1010 for a free case evaluation, or use the form on the right-hand side of your screen.today to schedule a free initial consultation.
Sources:
http://www.nejm.org/doi/full/10.1056/NEJM199506223322519
https://emedicine.medscape.com/article/1214410-treatment#d9
https://www.cdc.gov/mmwr/pdf/rr/rr62e0614.pdf