- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- Defective Drugs
- Defective Products
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
AndroGel therapy linked to possible health risks for men.
The health risks associated with AndroGel testosterone therapy have been well-publicized. Those risks include a number of cardiovascular issues, such as blood clots, DVT, heart attacks and strokes.
If you or someone you love was harmed as a result of AndroGel testosterone, you may be entitled to financial compensation. There may be substantial cash awards from claims and settlements from an AndroGel class action lawsuit or MDL. Call the lawyers and attorneys at National Injury Help today to see if you qualify for an AndroGel testosterone therapy lawsuit.
Call 1-800-214-1010 for a free case evaluation or use the form on the bottom of your screen.
Whatever the problems with the drug may be, they are apparently exacerbated by doctors who prescribe it for a host of ailments, prescriptions that ignore the limited extent of the drug’s approval by the U.S. Food and Drug Administration (FDA). This is called “off-label use” and this is not recommended by the FDA.
AndroGel Lawsuit Verdict – AbbVie sued for Misrepresentation of Risks
AbbVie a pharmaceutical company is based in Lake Bluff Illinois. It is the 6th largest pharmaceutical company in the world with posted revenue of $26 billion in 2016. It’s a spin-off that was created in 2013 of Abbott Laboratories.
Last July it had its day in a Chicago federal court where a jury found Abbvie Inc did in fact misrepresent the full risks of its testosterone therapy drug AndroGel. The drug maker was ordered to pay $150,000 million dollars in punitive damages. This verdict came to light from a lawsuit filed in 2014 from Jesse Mitchell and his wife. Currently there are over 6,000 AndroGel lawsuits lined up and consolidated in Chicago’s federal court system.
It’s interesting to note that the bases of this AndroGel lawsuit weren’t about the dangers of heart attack, stroke, blood clots or DVT problems. It was all about the way the drug was marketed. So the court didn’t award Mitchell any damages. “The jury found that AndroGel did not cause any damage,” an AbbVie spokesperson stated.
Last October AbbVie faced another jury and was sued for $140,000 million, again for misrepresentation of risks.
Past AndroGel Bellwether Lawsuits Claims.
Anytime you have a federal court award punitive damages without compensatory damages is rare. But this bellwether case means people harmed from AndroGel have a better chance of winning their claims. Here are the past AndroGel cases:
List of AndroGel Bellwether Cases by date of injury and type of alleged injury:
- 2008, alleged injury – Pulmonary embolism. Plaintiff: Arthur Myers V. Abbvie
- 2010, alleged injury – Heart attack. Plaintiff: Jeffrey Konrad V. Abbvie
- 2012, alleged injury – Pulmonary embolism. Plaintiff: Robert Nolte V. Abbvie
- 2012, alleged injury – Heart attack. Plaintiff: Jesse Mitchell V. Abbvie
- 2012, alleged injury – Heart attack. Plaintiff: Edward Cribbs V. Abbvie
- 2013, alleged injury – Deep vein thrombosis. Plaintiff: Robert Rowley V. Abbvie
- 2013, alleged injury – Stroke. Plaintiff: Cecile Frost V. Abbvie
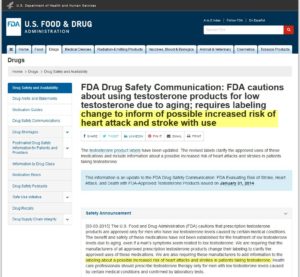
FDA Acts on AndroGel – Gets Black Box Warning Applied.
The first public warning for AndroGel was about possible transfer of the drug to children.
From the FDA website (link below) “The manufacturers of two prescription topical testosterone gel products, AndroGel 1% and Testim 1%, were required to include a boxed warning on the products’ labels. The agency required this action after receiving reports of adverse effects in children who were inadvertently exposed to testosterone through contact with another person being treated with these products (secondary exposure).”
The second public warning for AndroGel issued on January 31, 2014 was about the risk of stroke, heart attack, and death.
The starting point for this issue is to understand that the FDA approved the drug only for hypogonadism, that is, when the testicles do not produce enough testosterone. Low T therapy has not been approved for a myriad of other conditions, including age-related decline in testosterone levels, low sex drive, muscle loss, irritability or fatigue.
Do I Qualify for an AndroGel lawsuit? How do I file?
The best answer to see if you qualify for an AndroGel lawsuit is to simply call us at 1-800-214-1010 and speak directly to our intake staff; they will ask you a number of questions about your use of AndroGel. The attorneys and lawyers at National Injury Help can help you if you’ve been harmed by taking AndroGel.
Four common questions about the AndroGel lawsuit.
- How much can you get from an AndroGel lawsuit?
- I got blood clots or heart attack from AndroGel, can I sue Abbvie?
- How much compensation would I get from an AndroGel therapy lawsuit?
- How much are the settlement amounts in the AndroGel lawsuit?
The answer to these questions can be difficult to predict, as the class action or MDL for this hasn’t started yet. There is still time for join in this AndroGel lawsuit, but there are statutes of limitations that apply.
Some of the past defective drug lawsuits have had millions of dollars in a compensation fund, and then that is divided up between all who have joined the lawsuit. Example: $50,000,000 in a settlement fund that would serve 1,000 victims would be $50,000 per person. This is only an example and does not represent what may happen for AndroGel.
But as you can see from the above cases, Abbvie is paying out millions of dollars in court ordered settlements.
If you have taken AndroGel you may be entitled to compensation for any adverse health effects (blood clots, stroke, heart attack or DVT ) you may have suffered. No matter where you happen to live, contact National Injury Help to discuss the recovery of damages for your injuries. And if a loved one has died from AndroGel therapy, talk to us about the possibility of pursing a wrongful death claim.
AndroGel Therapy Lawsuit Claims & Settlements for Blood clots Heart attack stroke page updated on April 10, 2019