- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
-
-
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Cruise Ship Lawsuit Claims and Settlements
- Sexual Abuse Lawsuits
-
-
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
National Injury Help is currently investigating a link between the Bair Hugger Warming Blanket and bacterial infections. If you or someone you love has had knee or hip surgery and had an infection, contact our lawyers and attorneys today as there may be a class action lawsuit forming with substantial cash settlements from claims filed.
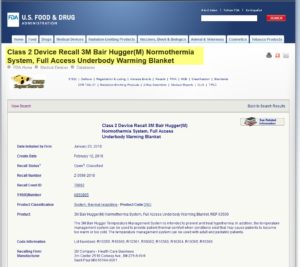
NEWS UPDATE: FDA has issued a mandatory Class 2 recall of 165,000 Bair Hugger Forced Air Warming Blankets on Feb. 12, 2018. Recalled lot numbers include these models: R10359, R10360, R10361, R10362, R10363, R10364, R10365 and R10366.
What are Bair Hugger Warming Blankets?
Warming blankets are commonly used when people have knee or hip surgery. The temperature management systems use forced heated air to keep people from getting hypothermia. But the problems lie in the fact that many of these units can lead to infections after having surgery. The system is basically a heater unit connected to a blower that leads to a porous blanket. Warm air circulates around and though the blanket.
Bair Hugger Therapy
It’s estimated that hospitals have treated more than 180 million people since the device came on the market in 1988. Dr. Scott D. Augustine the inventor of the device now speaks up on the device to let people know the risks. “I am very proud of the old technology,” he said. “But I am also proud to spread the word that there is a problem.” See the New Your Times article link at the bottom of this page.
The manufacturer now warns that the device shouldn’t be used for people having knee implants, hip implants, or heart valve surgery as the device may spread bacteria that may lead to infections. Infections happen when the blower pushes unclear hospital air around surgical wounds.
What are the problems or risks of the Bair Hugger Warming Blankets?
- Possible sepsis infections after knee or hip surgery. Various studies have shown a large increase of bacterial infestations of people who have been treated with the device.
- Possible loss of limb from amputations needed to treat the infections.
3M Bair Hugger and Arizant Relationship and the FDA
The FDA first approved the Bair Hugger Warming Blankets system in early 1987 under what is called the 510(k) program. This allows a medical manufacturer to produce devices without proof that it’s safe, if a similar device is already on the market.
3M purchased Arizant for over $800 million in 2010. Augustine Medical was the originator of the Bair Hugger device and changed the company name to Arizant. The product generated over $31 billion dollars in sales in 2014.
The FDA twice approved Arizant another 510(k) clearance to sell the product. As of today the FDA has not recalled the device, even though it’s aware of numerous people reporting problems with the device.
What is the Bair Hugger Warming Blanket Lawsuit about?
3M unarguably knew that the system could lead to infections in some people after surgery but didn’t warn hospitals or doctors about it. Failure to insure proper air filters were in place and failure to study the possible risk of infections.
Bair Hugger Lawsuits forming in these States:
Alabama (AL), Alaska (AK), Arizona (AZ), Arkansas (AR), California (CA), Colorado (CO), Connecticut (CT), Delaware (DE), Florida (FL), Georgia (GA), Hawaii (HI), Idaho (ID), Illinois (IL), Indiana (IN), Iowa (IA), Kansas (KS), Kentucky (KY), Louisiana (LA), Maine (ME), Maryland (MD), Massachusetts (MA), Minnesota (MN), Mississippi (MS), Missouri (MO), Montana (MT), Nebraska (NE), Nevada (NV), New Hampshire (NH), New Jersey (NJ), New Mexico (NM), New York (NY), North Carolina (NC), North Dakota (ND), Ohio(OH), Oklahoma (OK), Oregon (OR), Pennsylvania (PA), Rhode Island (RI), South Carolina (SC), South Dakota (SD), Tennessee (TN), Texas (TX), Utah (UT), Vermont (VT), Virginia (VI), Washington (WA), Washington DC (DC), West Virginia (WV), Wisconsin (WI), Wyoming (WY)
Our lawyers and attorneys can provide information on how to file a Bair Hugger lawsuit in the following cities in our claims center:
New York, Chicago, Philadelphia, Detroit, Indianapolis, Columbus, Baltimore, Boston, Seattle, Washington, Milwaukee, Denver, Louisville, Los Angeles, Las Vegas, Nashville, Oklahoma City, Portland, Phoenix, Houston, Tucson, Albuquerque, Atlanta, San Antonio, San Diego, San Francisco, Dallas, Colorado Springs, Arlington, Wichita, Long Beach, Fresno, Sacramento, Mesa, Kansas City, Cleveland, San Jose, Jacksonville, Austin, Memphis, Fort Worth, Charlotte, Virginia Beach, Omaha, Miami, Oakland, Tulsa, Honolulu, Minneapolis.
Source: https://www.nytimes.com/2010/12/25/business/25invent.html?_r=0
Bair Hugger Infection lawsuits claims & settlements page updated on April 5, 2019 for FDA Recall on Bair Hugger warming blankets.