- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- Defective Drugs
- Defective Products
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
The defective drug lawyers and attorneys at National Injury Help are now accepting Zinbryta lawsuit claims for US citizens who have had liver damage, liver failure, brain inflammation or damage from taking the self administered MS drug Zinbryta. There may be substantial cash payouts from settlements in this class action lawsuit.
Sales of this drug worldwide brought in $107 million in 2017. Biogen reported total revenues of 3.1 billion in 2018.
What is Zinbryta?
Daclizumab (trade name Zinbryta) is a humanized monoclonal antibody drug that is once-monthly self-injected into the persons thigh muscle for treatment of relapsing multiple sclerosis (MS). Zinbryta was first approved by the FDA in 1997 and used to prevent acute rejection of kidney transplants.
The second FDA approval took place in May 2016 (including a boxed warning due to risks of liver damage) for the treatment of relapsing multiple sclerosis in adults. It’s estimated that over 8,000 patients have been treated with Zinbryta worldwide.
Zinbryta was brought to market by AbbVie Inc for sale in America. Biogen is the seller in Switzerland, Canada and European Union. Since it was the first of its kind, a humanized antibody, its annual sales were forecasted to be between $100 million and $250 million.
Zinbryta Recall – Removed from the Market.
Last November European regulators voiced safety concerns about the risks of possible serious liver damage. Biogen pulled Zinbryta from the market April 30, 2018 because of potential adverse events such as liver damage, inflammatory brain disease, encephalitis, seizures, and even death.
Biogen’s executive vice president and chief medical officer Dr. Alfred Sandrock issued this statement: “Biogen believes the voluntary worldwide withdrawal of Zinbryta, a treatment for relapsing multiple sclerosis, is in the best interest of patients.” He further states: “Biogen and AbbVie continue to prioritize patient safety and the care of multiple sclerosis patients worldwide.”
The company explained in its press release that it will work with the FDA and EMA as well as doctors worldwide in removing Zinbryta from the market.
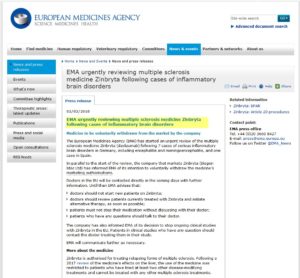
The European Medicines Agency (EMA), the EU version of our FDA, issued a press release on February 3, 2018; the following is a direct excerpt from it:
EMA urgently reviewing multiple sclerosis medicine Zinbryta following cases of inflammatory brain disorders
Medicine to be voluntarily withdrawn from the market by the company
The European Medicines Agency (EMA) has started an urgent review of the multiple sclerosis medicine Zinbryta (daclizumab) following 7 cases of serious inflammatory brain disorders in Germany, including encephalitis and meningoencephalitis, and one case in Spain.
In parallel to the start of the review, the company that markets Zinbryta (Biogen Idec Ltd) has informed EMA of its intention to voluntarily withdraw the medicine’s marketing authorisations.
Doctors in the EU will be contacted directly in the coming days with further information. Until then EMA advises that:
- doctors should not start new patients on Zinbryta;
- doctors should review patients currently treated with Zinbryta and initiate alternative therapy, as soon as possible;
- patients must not stop their medication without discussing with their doctor;
- patients who have any questions should talk to their doctor.
The company has also informed EMA of its decision to stop ongoing clinical studies with Zinbryta in the EU. Patients in clinical studies who have any question should contact the doctor treating them in their study. EMA will communicate further as necessary.
————–END OF PRESS RELEASE———–
Zinbryta Deaths & Brain Inflammation.
As noted above, it was the EMA that alerted the medical community after a report of death from liver failure (fulminant liver failure) of a patient being treated with Zinbryta in an ongoing study. Four others in this study group suffered serious liver injury. There have been 12 known reports of brain inflammation linked to this MS drug in the US.
What are the Zinbryta Side Effects?
The safety of the multiple sclerosis medicine Zinbryta is being reviewed; however we were able to find some of the known side effects which may include the following:
- liver problems
- fulminant liver failure
- brain inflammation
- encephalitis
- hepatic injury
- unexplained nausea
- vomiting
- abdominal pain
- tiredness
- loss of appetite
- yellowing of the skin and eyes and dark urine
Zinbryta Class Action Lawsuit for Liver Damage, Brain Inflammation
If you suffer from multiple sclerosis and have been prescribed Zinbryta and had liver damage, brain swelling or inflammation, we can help you protect your rights. Take a moment to call our Zinbryta lawyers at National Injury Help by calling 1-800-214-1010, or use the form on this page. There is never a cost or fee to get the answers you deserve. The upcoming Zinbryta class action lawsuit may be starting soon. Act now.
Sources:
https://www.fda.gov/Drugs/DrugSafety/ucm600999.htm
https://www.medscape.com/viewarticle/893352
https://www.reuters.com/article/us-biogen-zinbryta/biogen-abbvie-withdraw-multiple-sclerosis-drug-zinbryta-idUSKCN1GE1BV
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2017/07/news_detail_002773.jsp&mid=WC0b01ac058004d5c1
Zinbryta Death, Liver Damage & Brain Inflammation Class Action Lawsuit page updated on April 5, 2019