- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Birth Injuries
- Commercial Fishing Boat Lawsuit Claims and Settlements
- Cruise Ship Lawsuit Claims and Settlements
- Nursing Home Abuse
- Medical Malpractice
- Sexual Abuse Lawsuits
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
People living with mental health disorders like schizophrenia and Bipolar disorder are often prescribed medications to help treat or alleviate the symptoms of their disorders. Risperdal is a popular antipsychotic drug used to treat these and other disorders in both adults and children. Now, some people are speaking out against the drug, saying it caused serious side effects like the development of male breasts. Learn more about Risperdal and the possible side effects that have over thousand former users filing lawsuits against the drugmaker.
What is Risperdal?
Risperdal is the brand name of an antipsychotic medication used to treat certain mental health disorders. The main ingredient in Risperdal, risperidone, belongs to a class of drugs known as atypical antipsychotics, or second generation antipsychotics.
What is Risperdal used for?
Risperdal is indicated for the treatment of schizophrenia, acute manic or mixed episodes associated with Bipolar I Disorder, and irritability associated with autistic disorder.
Risperdal has also been used “off-label” to treat other disorders, including Obsessive Compulsive Disorder (OCD), dementia and Alzheimer’s disease, Attention Deficit Hyperactive Disorder (ADHD), Tourette’s syndrome and Asperger’s disorder.
Risperdal, like some other atypical antipsychotics, has mood stabilizing effects which help treat patients with intense and sustained mood shifts. Because of this, doctors may prescribe Risperdal to some patients even if psychotic symptoms are not present.
Risperdal is approved for use in adults, as well as children diagnosed with certain disorders. Risperdal has not been approved for use in elderly patients with dementia-related psychosis. Other uses of Risperdal by elderly patients have not been sufficiently studied.
How does Risperdal work?
Like all atypical antipsychotic drugs, Risperdal’s exact mechanism of action is unknown. What is known is that the drugs work by blocking a specific dopamine receptor in the brain, the dopamine D2 receptor.
Dopamine is a naturally occurring chemical that plays important roles in the brain and throughout the body. In the brain, dopamine acts as a neurotransmitter, sending signals to other nerve cells. Dopamine plays a major role in reward-motivated behavior, as well as motor control and controlling the release of various hormones. D2 receptors are one of the most abundant dopamine receptors in the brain.
Who manufactures Risperdal?
Risperdal is manufactured and marketed by Janssen Pharmaceuticals Inc., a subsidiary of Johnson & Johnson.
Janssen lost its patent and exclusive marketing rights over Risperdal in 2003 and 2004, respectively. Now, generic versions of the drug are available and sold by more than a dozen other pharmaceutical companies.
When did Risperdal come on the market?
The Food and Drug Administration (FDA) approved Risperdal on Dec. 29, 1993. The drug was initially approved to treat schizophrenia in adults. It was later approved to treat certain symptoms of Bipolar I Disorder and autism.
Are there generic versions of Risperdal available?
Yes. Janssen Pharmaceuticals lost its patent for risperidone on December 23, 2003. Since then, more than a dozen companies have received FDA approval to sell a generic version of Risperdal under the generic name risperidone.
Generics are generally cheaper than their name-brand alternatives and contain the same active ingredients.
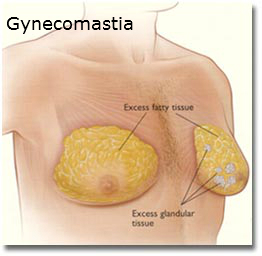
What is gynecomastia?
Gynecomastia is a possible side effect of Risperdal. Gynecomastia is the overdevelopment of the male breast, usually caused by too much estrogen or too little testosterone. The condition causes boys and men to develop larger than normal breasts and can cause serious emotional trauma to the sufferer.
Symptoms of gynecomastia include:
- swollen breast gland tissue
- breast tenderness
- pain
- nipple discharge
Boys and men suffering with gynecomastia may choose to have surgery to remove the breast tissue. Liposuction removes the breast fat, but not the breast gland tissue itself; a mastectomy removes the breast gland tissue.
How does Risperdal cause gynecomastia?
Risperdal increases the level of a protein in the body called prolactin. Risperdal increases prolactin levels more so than other antipsychotics.
Prolactin is responsible for the production of breast milk in mammals; higher than normal levels of prolactin in the blood can trigger gynecomastia in boys and men.
Several studies indicate Risperdal may contribute to the development of gynecomastia, especially in young boys.
A Swiss study published in 2006 in the Journal of Clinical Psychopharmacology found risperidone, the active ingredient in Risperdal, can strongly increase prolactin levels and cause gynecomastia. The authors of the study found that of the 10 adolescents treated in their unit with risperidone, three boys developed gynecomastia. Almost all of the adolescents (eight out of 10) had elevated levels of prolactin in their blood, though not all showed symptoms.
The study concluded that “risperidone should be administered with caution to children and adolescents.”
A Canadian study published in the Journal of Child and Adolescent Psychopharmacology last year came to a similar conclusion. The study found that males between the ages of 15 and 25 were four times more likely to develop gynecomastia compared to 15 to 25 year olds not taking risperidone. When the study authors limited the group to males aged 15 to 18, they found those teens were five times more likely to develop gynecomastia when taking risperidone.
What other possible side effects are associated with Risperdal?
Along with gynecomastia, Risperdal carries the risk of potential side effects that range from mild to severe.
As reported by Drugs.com, relatively mild side effects that have been reported by patients using Risperdal include:
More common:
- constipation
- cough
- diarrhea
- dry mouth
- headache
- heartburn
- increased dream activity
- increased length of sleep
- nausea
- sleepiness or unusual drowsiness
- sore throat
- stuffy or runny nose
- unusual tiredness or weakness
- weight gain
Less common:
- absent, missed, or irregular menstrual periods
- body aches or pain
- breast swelling or soreness
- chills
- dandruff
- darkening of skin color
- decreased interest in sexual intercourse
- dry skin
- ear congestion
- fever
- inability to have or keep an erection
- increase in body movements
- increased watering of the mouth
- joint pain
- loss in sexual ability, desire, drive, or performance
- loss of voice
- oily skin
- pain or tenderness around the eyes and cheekbones
- shortness of breath or troubled breathing
- sneezing
- stomach pain
- stopping of menstrual bleeding
- tightness of the chest or wheezing
- toothache
- unusual breast milk production
- vomiting
- weight loss
More severe side effects reported by patients using Risperdal include:
More common:
- aggressive behavior
- agitation
- anxiety
- changes in vision, including blurred vision
- difficulty concentrating, racing thoughts
- difficulty speaking or swallowing
- inability to move the eyes
- increase in amount of urine or frequent urination
- loss of balance control
- mask-like face
- memory problems, like short-term memory loss
- muscle spasms of the face, neck, and back
- problems with urination
- restlessness or need to keep moving (severe)
- shuffling walk
- skin rash or itching
- stiffness or weakness of the arms or legs
- tic-like or twitching movements
- trembling and shaking of the fingers and hands
- trouble sleeping, insomnia
- twisting body movements
Less common:
- back pain
- chest pain
- speech or vision problems
- sudden weakness or numbness in the face, arms, or legs
Rare:
- confusion
- dizziness
- drowsiness
- extreme thirst
- fast, shallow breathing
- fast, weak heartbeat
- headache
- increased thirst
- lip smacking or puckering
- loss of appetite
- muscle cramps
- pale, clammy skin
- poor coordination
- prolonged, painful, inappropriate erection of the penis
- puffing of the cheeks
- rapid or worm-like movements of the tongue
- shivering
- talking, feeling, and acting with excitement and activity that cannot be controlled
- uncontrolled chewing movements
- uncontrolled twisting movements of neck, trunk, arms, or legs
- unusual bleeding or bruising
- unusual facial expressions or body positions
Which drugs can interact with Risperdal?
Certain drugs may cause adverse reactions when used in combination with Risperdal. According to the drug’s label, the following drugs should not be taken with Risperdal:
- Cimetidine (Tagamet) and Ranitidine (Zantac, Select)
- Clozapine (Clozaril and FazaClo)
- Valproate (Depakote, Depakene, Stavzor, Valproic)
- Topiramate (Topamax, Qudexy)
- Drugs that inhibit CYP 2D6 and other CYP Isozymes, including some SSRIs
- Cabamazepine (Tegretol, Carbatrol, Equetro, Epitol)
The following drugs should not be used in combination with Risperdal Consta:
- Centrally-acting drugs (drugs that lower heart rate and reduce blood pressure, such as Catapres, Tenex and methyldopa)
- Alcohol
- Drugs with hypotensive effects
- Levodopa and dopamine agonists, such as Mirapex, Cycloset and Apokyn
Risperdal is also contraindicated in the following patients:
- Patients with a known hypersensitivity to either risperidone or paliperidone (a metabolite of risperidone), or to any of the ingredients in Risperdal.
How do you take Risperdal?
Risperdal is available as a tablet, an oral solution and an orally disintegrating tablet (ODT) sold under the trade name Risperdal M-Tab.
Risperdal Tablets are available in multiple strengths and each pill is a different color: 0.25 mg (dark yellow), 0.5 mg (red-brown), 1 mg (white), 2 mg (orange), 3 mg (yellow) and 4 mg (green). All tablets are capsule-shaped. Risperdal used to be available in 5 mg strength tablets, but that strength was discontinued.
Risperdal Oral Solution is only available in a 1 mg/mL strength. Risperdal Oral Solution can be taken directly from the calibrated pipette or mixed with a beverage (water, coffee, orange juice or low-fat milk).
Risperdal M-TAB is available in multiple strengths, colors and shapes: 0.5 mg (light coral, round), 1 mg (light coral, square), 2 mg (coral, square), 3 mg (coral, round) and 4 mg (coral, round).
The prescribed dosage amount will vary from patient to patient depending on his or her age and the disorder being treated, among other factors. The tablets, oral solution and ODT are usually taken daily, unless otherwise specified by a doctor.
Risperdal Consta
In 2003, the FDA approved Risperdal Consta, an intramuscular (IM) injection available in 12.5 mg, 25 mg, 37.5 mg and 50 mg vials.
Risperdal Consta is given as a shot into the arm or buttocks. Unlike other forms of Risperdal which are usually taken daily, Risperdal Consta is administered every two weeks. The maximum dose should not exceed more than 50 mg every two weeks.
Risperdal overdose
It is possible to overdose on Risperdal in any form. Reports of Risperdal overdose include doses from 20 mg up to 360 mg. Signs and symptoms of overdose may include:
- Drowsiness
- Sedation
- Tachycardia
- Hypotension
- Extrapyramidal symptoms (EPS), like unwanted movements, muscle breakdown, tremors, seizures
A few reported cases of overdose also included the following symptoms:
- Seizure
- QT prolongation (fast, chaotic heartbeat)
- Hyponatremia (low sodium in the blood)
- Hypokalemia (low potassium in the blood)
Risperdal withdrawal
Risperdal may be difficult to stop taking. Do not stop taking Risperdal without first speaking with your doctor. The following is a list of withdrawal symptoms reported by patients after stopping Risperdal:
- Agitation
- Anorexia
- Anxiety
- Diarrhea
- Dizziness
- Vomiting
- Extrapyramidal symptoms (EPS), like unwanted movements, muscle breakdown, tremors, seizures
- Hyperkinesis (muscle spasms)
- Insomnia
- Itching
- Muscle pain
- Nausea
- Restlessness
- Runny nose
- Somnolence (sleepiness or drowsiness)
- Sweating
How much does Risperdal cost?
The price of Risperdal varies, especially depending on the dosage needed. The following prices are available at Drugs.com using the Drugs.com discount card without insurance:
- 25 mg – $499 for 60 tablets
- 5 mg – $180 for 30 tablets
- 1 mg – $210 for 30 tablets
- 2 mg – $346 for 30 tablets
- 3 mg – $482 for 30 tablets
- 4 mg – $542 for 30 tablets
There are also generic versions of the drug available. Generics are generally cheaper than the name brand drug and have the same active ingredients. The following are prices for the generic risperidone taken from Drugs.com using the Drugs.com discount card without insurance.
- 25 mg – $110 for 60 tablets
- 5 mg – $88 for 50 tablets
- 1 mg – $22 for 30 tablets
- 2 mg – $61 for 30 tablets
- 3 mg – $99 for 50 tablets
- 4 mg – $160 for 50 tablets
Insurance may cover the cost of your Risperdal or risperidone prescription. Janssen Pharmaceuticals also offers special assistance programs to Risperdal patients who qualify.
Are there comparable drugs to Risperdal on the market?
Several other atypical antipsychotics, or second generation antipsychotics, are available on the market with a prescription. These include:
- Aripiprazole (Abilify)
- Asenapine (Saphris)
- Clozapine (Clozaril, FazaClo)
- Iloperidone (Fanapt)
- Lurasidone (Latuda)
- Olanzapine (Zyprexa)
- Paliperidone (Invega)
- Quetiapine (Seroquel)
- Ziprasidone (Geodon)
Not all atypical antipsychotics are approved to treat the same mental health disorders and will affect people in different ways.
Is Risperdal safe during pregnancy or while breastfeeding?
Pregnant women
Risperdal is a pregnancy category C drug. This means animal studies have shown adverse effects on the fetus, but no adequate testing has been done in humans.
Though Risperdal has not been studied in pregnant women, animal testing showed fetuses exposed to the drug during the third trimester were at risk for extrapyramidal (EPS) signs and withdrawal symptoms after being born. The risk of death was higher for pups exposed to all doses of Risperdal in peri-postnatal studies in rats.
The makers of Risperdal warn the drug should only be taken during pregnancy if the benefits outweigh the risks.
Nursing mothers
The drugmaker warns nursing mothers should not take Risperdal because the drug can pass through breast milk to the infant. New mothers who take Risperdal should either discontinue the drug or discontinue breastfeeding. This decision should take into account the importance of the drug to the mother, according to the Risperdal label.
Has the FDA issued any warnings about Risperdal?
Yes, the FDA has issued several safety warnings to the public regarding Risperdal.
“Black Box” Warning
In 2005, the FDA issued a warning about Risperdal and the increased risk of death in elderly patients with dementia. The FDA said the treatment of behavioral disorders in elderly patients with dementia with Risperdal and other atypical antipsychotics increased a patient’s risk of death by nearly two-fold.
As a result, the FDA issued a “black box” warning for all atypical antipsychotics regarding the increased risk of death in elderly patients with dementia. “Black box” warnings are the FDA’s strongest warnings, indicating there is an increased risk of serious injury or death associated with the drug.
Risk to unborn babies
In 2011, the FDA issued a safety announcement about the possible risk of abnormal muscle movements (extrapyramidal, EPS, signs) and withdrawal symptoms in newborns whose mothers were treated with Risperdal during the third trimester of pregnancy. The drug’s label was updated to include this risk.
Medication mix up
The FDA issued a safety communication to patients and doctors in 2011 warning them about the possibility of mixing up Risperdal with another medication.
The agency received several reports of patients receiving ropinirole (marketed as Requip), a dopamine promoter used to treat Parkinson’s disease, instead of risperidone (Risperdal) and vice versa. Some of those patients were hospitalized as a result of the mix up.
The FDA determined the following factors contributed to the confusion:
- Similarities of both brand and generic names
- Similarities of the container labels and carton packaging
- Illegal handwriting on prescriptions
- Overlapping product characteristics, such as drug strengths, dosage forms and dosing intervals
Has Risperdal been recalled?
Janssen Pharmaceuticals voluntarily recalled about 16,000 bottles of Risperdal 2 mg and 3 mg oral tablets in June 2011 due to an unusual odor. The company believed trace amounts of TBA had contaminated the products. TBA is a byproduct of a chemical used to preserve wood pallets.
Apart from this specific recall, Risperdal has not been taken off the market and is still being prescribed to patients worldwide.
Have lawsuits been filed against the makers of Risperdal?
Yes, over 1,500 lawsuits have been filed against Janssen Pharmaceuticals, the makers of Risperdal, and its parent company Johnson & Johnson, alleging the companies failed to warn consumers about the potential risks associated with the drug. These lawsuits have been consolidated in a mass tort program in the Philadelphia Complex Litigation Center. Unlike a class action lawsuit, these cases are being heard individually by the court.
Have any settlements been made in Risperdal cases?
Yes. Johnson & Johnson agreed to settle one Risperdal case in 2012, an amount that remains confidential.
Four other Risperdal cases have gone to trial, with juries handing down three verdicts in favor of the plaintiffs and one in favor of Johnson & Johnson.
Risperdal cases have ended with substantial compensation for plaintiffs. Jurors awarded $2.5 million in February 2015 to a man who developed breasts while taking Risperdal as a child. A judge recently upheld the $2.5 million decision after J&J appealed. In November 2015, a Maryland man was awarded $1.75 million, which was later reduced to $680,000; and in December 2015, a Wisconsin man was awarded $500,000.
In March 2015, jurors found Janssen negligent in warning about the risks of Risperdal but failed to connect the plaintiff’s abnormal breast growth with Janssen’s negligence.
Janssen vs. Federal Government
In 2013, Janssen Pharmaceuticals agreed to pay more than $1.39 billion as part of a settlement to resolve allegations the company illegally marketed Risperdal for unapproved uses.
The lawsuit alleged Janssen began marketing Risperdal to treat certain disorders in children and the elderly even though the drug had not been approved by the FDA for those uses. The FDA would later approve the use of Risperdal to treat children with certain disorders but not the elderly.
The lawsuit also alleged both Janssen and its parent company Johnson & Johnson knew of the risks that Risperdal posed to children and the elderly but the companies downplayed those risks.
In total, J&J paid $2.2 billion to the Department of Justice to settle criminal and civil allegations involving Risperdal and other medications.
I took Risperdal as a child and developed male breasts, can I file a lawsuit?
If you took Risperdal as a young boy and developed male breasts you may be entitled to compensation. Call the National Injury Attorneys, LLC today to speak with our experienced lawyers and attorneys. They can help you determine if you have a case and seek the justice you deserve. With so many lawsuits already pending in court, you need to act now. Call us today at 1-800-214-1010 for a free case evaluation or use the form on the right-hand side of your screen.