- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
-
-
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Cruise Ship Lawsuit Claims and Settlements
- Sexual Abuse Lawsuits
-
-
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
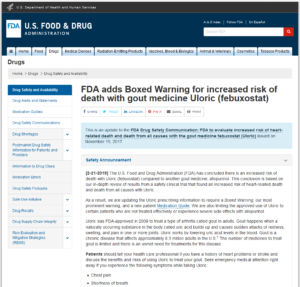
Uloric Warning issued by the FDA – Uloric may increase risk of heart attacks and heart related deaths.
The lawyers and attorneys at National Injury Help are now investigating the drug Uloric (febuxostat) and its possible risk of heart related injury, heart attack and heart related deaths from this drug. There may be substantial cash settlements from these Uloric claims.
Consumer warning: If you are currently taking Uloric DO NOT STOP taking your medication, ask your doctor first.
What is Uloric used for?
Febuxostat, sold under the brand name Uloric, is a common drug used to treat a type of arthritis called gout. Gout is a condition that causes extreme pain, redness and swelling (inflammation) in joints. It is caused by high levels of uric acid in the kidneys that turns into needle shaped crystals deposited in the joints. Most common areas affected are the big toe joint, knees and ankles. Men suffer this condition more often than women.
In a recent study FDA’s Arthritis Advisory Committee and the Drug Safety and Risk Management Committee, found a higher mortality risk compared to another common gout drug allopurinol. The agency now states that Uloric should only be prescribed for patients who have tried allopurinol first.
Public Agency asks FDA to remove Uloric from the market.
Public Citizen, a nonprofit consumer advocacy organization, urged the committee to recommend that the FDA immediately remove from the market the widely used gout medication febuxostat because it poses unique, serious risks – include the risk of dying from cardiovascular disease – but offers no unique benefit. Public Citizen had requested such action in a citizen petition submitted to the agency on June 21, 2018.
FDA Black Box Warning Issued for Uloric. Has Uloric been Recalled?
Excerpt: [2-21-2019] The U.S. Food and Drug Administration (FDA) has concluded there is an increased risk of death with Uloric (febuxostat) compared to another gout medicine, allopurinol. This conclusion is based on our in-depth review of results from a safety clinical trial that found an increased risk of heart-related death and death from all causes with Uloric.
As a result, we are updating the Uloric prescribing information to require a Boxed Warning, our most prominent warning, and a new patient Medication Guide. We are also limiting the approved use of Uloric to certain patients who are not treated effectively or experience severe side effects with allopurinol.
The FDA-mandated study, published in The New England Journal of Medicine in 2018, revealed that the “treatment with Uloric resulted in overall rates of major cardiovascular events that were similar to those associated with Allopurinol treatment among patients with gout who had coexisting cardiovascular disease. However, cardiovascular death and deaths from any cause were more frequent in the Uloric group than in the Allopurinol group”.
At this time the FDA is not recalling Uloric from the market.
Help Filing an Uloric Lawsuit, Class action Status.
If you or someone you love has been prescribed the drug Uloric and has suffered any heart related injuries, heart attack or even death we can help. You may be eligible for a large cash compensation for your injuries. We make it easy filing an Uloric lawsuit, simply use the contact form on the bottom of this page or call us directly at 1-800-214-1010. We will never charge a fee for this consultation.
Join in the free Uloric Class Action Status & Investigation – MDL for Uloric
At the time of this writing the class action status of Uloric is undetermined. However we feel strongly that there is a case against Takeda Pharmaceuticals and it may in fact be granted an MDL (Multi District Litigation) status by the courts. Back in 2012 a former safety employee of Takeda came forth and made statements about the drug’s side effects and was granted a whistleblower lawsuit.
Our law firm is closely watching this new FDA warning and helping any person who may have been harmed by Uloric.
Sources:
https://www.fda.gov/Drugs/DrugSafety/ucm631182.htm
Uloric Lawsuit Attorneys & Lawyers | Claims & Settlements for Uloric page updated on 2/27/2019