- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Birth Injuries
- Commercial Fishing Boat Lawsuit Claims and Settlements
- Cruise Ship Lawsuit Claims and Settlements
- Nursing Home Abuse
- Medical Malpractice
- Sexual Abuse Lawsuits
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
Essure has been making headlines ever since thousands of women began speaking out against the permanent form of birth control and the serious side effects it can potentially cause. Learn more about the controversial contraceptive that has both patients and lawmakers fighting to get it pulled from the market.
What is Essure?
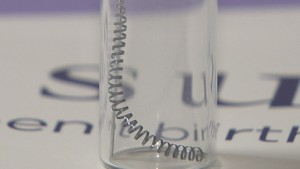
Essure is a form of permanent birth control (sterilization) that involves placing two metal coils in a woman’s fallopian tubes. Essure is the only non-surgical form of permanent contraception available today. The procedure is performed in a doctor’s office usually in less than 30 minutes.
How does Essure work?
Essure works by blocking the fallopian tubes so sperm cannot reach an egg to fertilize it. The Essure device consists of two metal coils that are inserted into each fallopian tube by a doctor. The coils cause the body to create scar tissue, which blocks sperm from entering the fallopian tubes. This process is intended to be permanent and usually cannot be reversed; women who may want more children in the future should not get Essure.
How is the Essure insertion procedure performed?
Essure is inserted into each fallopian tube by a doctor. The coils are inserted through the vagina so no incision is necessary. The doctor first inserts a small camera called a hysteroscope through the vagina, into the cervix and up the uterus until he or she can see the fallopian tubes. The doctor then passes the Essure coils through the hysteroscope and into the fallopian tubes.
The correct placement is essential in order for Essure to work as intended. About three to eight rings should extend into the uterine cavity; the rest of the coils should be inside the fallopian tubes.
How long before Essure is effective as birth control?
It usually takes about three months after initial insertion of the Essure device for the fallopian tubes to become completely blocked by scar tissue. The makers of Essure say it can take up to six months for some women.
Until the fallopian tubes are completely closed, women must use an alternative form of birth control, like condoms, to prevent pregnancy.
To ensure the fallopian tubes are completely blocked, women must revisit their doctor for a hysterosalpingogram, or HSG.
What is an HSG (hysterosalpingogram) procedure?
A hysterosalpingogram, or HSG, is an x-ray procedure used to see if the fallopian tubes are open. This test is usually performed about three months after the insertion of Essure. During the HSG procedure, a doctor fills the uterus with dye, which flows from the uterus into the fallopian tubes. If the fallopian tubes are still open, the dye will travel to the ovaries. Until the fallopian tubes are completely closed, women need to use an alternative form of birth control to prevent pregnancy.
What is Essure made of?
The Essure device consists of two small metal coils that are placed inside each fallopian tube. The metal coils are made of an outer nickel titanium alloy coil and an inner stainless steel coil. Wrapped around the inner stainless steel coil are PET (polyethelene) fibers. The PET fibers stimulate the growth of scar tissue by acting as an irritant to the fallopian tubes. The inner coil holds the PET fibers in place while the outer coil is intended to act as an anchor and hold the entire device in the fallopian tubes.
Why do women get Essure?
When women no longer want to have children, they may consider several different options of permanent birth control. Essure is the only non-surgical method of permanent birth control available. Many women are attracted to Essure because it is advertised as a quick, easy procedure with little to no down time.
Are there other options of permanent birth control available?
While Essure is the only non-surgical method of permanent birth control, there are other methods that require surgery for both women and men.
Tubal ligation, also known as getting one’s tubes tied, is a surgical option of permanent birth control which doctors have performed on hundreds of thousands of women since the early 20th century. Tubal ligation involves cutting, burning or clamping the tubes closed so that sperm cannot enter the tubes and fertilize an egg. Tubal ligation is a usually performed in a hospital operating room setting and is considered a major surgery. Women must undergo spinal anesthesia to get the surgery.
While tubal ligation is considered permanent, tubal reversal is possible if a woman decides she wants to have another child. The success rate of tubal reversal depends on many factors, including the woman’s age and the type of tubal ligation she had.
About 700,000 tubal ligations are performed each year in the United States.
Men can opt to have a vasectomy to prevent his partner from becoming pregnant. A vasectomy is a minimally invasive surgical procedure that involves cutting the vas deferens so sperm cannot enter the seminal fluid. Vasectomies are usually performed in a doctor’s office in less than 30 minutes.
About 500,000 vasectomies are performed in the United States each year.
What are the side effects of Essure?
Essure has been linked to a multitude of adverse side effects by thousands of women who had the metal coils inserted. Side effects can range from mild to severe; some may even be life-threatening. The following symptoms and side effects have been reported by women who had Essure inserted:
- Heavy or irregular menstrual cycles
- Fatigue
- Weight gain or loss
- Allergies to nickel
- Pregnancy, ectopic pregnancy
- Device migration
- Perforation of organs
- Broken or missing coils
- Chronic pelvic pain
- Pain – lower back, hip, joint, chest, leg, neck, spine
- Hair loss
- Hair growth in new places
- Headaches or migraines
- Nausea, vomiting
- Malaise, general feeling of illness or discomfort
- Severe bloating
- Cramping
- Endometriosis
- Fibroids
- Night sweats
- Loss of libido
- Bleeding/spotting after sex
- Painful intercourse (dyspareunia)
- Yeast infections
- Bacterial vaginosis
- Urinary tract infections (UTIs) or bladder infections
- Blood in urine
- Swelling, inflammation of cervix or vagina
- Itching, burning, stabbing pain at vaginal opening
- Breast pain, tenderness
- Constipation, gas, diarrhea
- Metallic taste in mouth
- Heartburn
- Mood swings
- Dizziness
- Anxiety
- Depression
- Tingling sensations
- Brain fog, forgetfulness
- Fainting/black out spells
- Blood clots
- Vitamin D or B12 deficiencies
- Anemia, iron deficiency
- Hives, rashes, skin irritation
- Acne
- Dental issues
- Insomnia
- Thyroid disease
- Swelling of legs, feet
- Muscle spasms
- Vision problems
- Excessive sweating
- Dry skin, hair or eyes
What is migration of the device?
Device migration is a relatively common adverse events reported by women who have problems with the device. Of the 9,900 adverse event reports sent to the FDA between 2002 and 2015, more than 800 included migration of the device.
When the Essure device migrates, it means the device moves from the proper place of insertion inside the fallopian tubes. Sometimes, the device moves only slightly from its intended position; in other cases, the device may migrate completely out of the fallopian tube into the uterus or the abdominal cavity. This usually happens when the doctor inserts the coils too far into the fallopian tube or not far enough.
Symptoms of device migration include:
- Abnormal bleeding
- Abdominal pain
- Lower back pain
- Tiredness
- General feeling of discomfort, illness
- Nausea, vomiting
- Headaches
- Fatigue
- Anxiety
Can Essure fail?
Yes. Essure has been linked to hundreds of unplanned pregnancies, in some cases after a woman has undergone an HSG to ensure the tubes are closed.
While no form of birth control is 100 percent effective all of the time, women may be at a greater risk of becoming pregnant with Essure than tubal ligation. One study published in the journal Contraception estimated that 9.6 percent of women could become pregnant within 10 years of undergoing the Essure procedure. That is about four times the risk after tubal ligation.
Between 2002 and 2015, over 630 pregnancies were reported to the FDA by women who had Essure inserted but became pregnant anyway.
Women who have Essure inserted and subsequently become pregnant are at a greater risk for ectopic pregnancies. Ectopic pregnancies occur when the fertilized egg implants itself anywhere except the uterus. Oftentimes, the fertilized egg implants itself in the fallopian tubes. Ectopic pregnancies are serious and life-threatening to both mother and baby. In most cases the baby does not survive.
Can Essure be removed?
Women suffering from adverse events related to Essure often want the coils removed. On their journey to becoming “E-free,” many women may be shocked and disappointed to learn that Essure must be surgically removed. Women may need to undergo full hysterectomies to get all of the coils out.
Essure removal surgery must be performed by an experienced surgeon as the device can be difficult to remove. The coils can easily break apart when surgeons attempt to remove them, risking more complications and side effects if fragments are left behind. The device’s tendency to migrate after placement can also present a challenge to the surgeon.
There are several procedures a surgeon can use to remove the Essure coils. Which procedure the surgeon chooses will depend on many factors, including his or her own experience removing Essure.
The following procedures are often used to remove Essure:
- Salpingotomy – A surgeon makes an incision in the fallopian tubes and removes the coils intact. There is a risk of fragmentation with this procedure. It must be performed by a specialist with extensive experience.
- Total hysterectomy with bilateral salpingectomy – Unfortunately, most women who have the coils removed must undergo a total hysterectomy. A total hysterectomy involves removing the uterus and cervix. A bilateral salpingectomy involves removing the fallopian tubes, as well. Hysterectomies still carry the risk of fragmentation of the device. Other risks of hysterectomies include early menopause and pelvic organ prolapse.
Can Essure be reversed?
Essure is intended to be a permanent form of birth control and is extremely difficult to remove. For women who want to become pregnant after undergoing the Essure procedure, it is especially difficult.
The reversal procedure involves carefully cutting the fallopian tubes above the Essure coils and removing the coils intact along with surrounding scar tissue. The tubes are then re-inserted into the uterus to function as they normally would pre-sterilization. All of this is done through a small incision in the abdomen just above the pubic bone.
There are relatively few doctors who specialize in Essure reversal in the United States. One tubal reversal center estimates of the 143 Essure reversals it has performed since 2008 about 40 percent of women were able to conceive after the procedure.
Are there doctors in my area who can perform an Essure removal procedure?
It is important to seek out a doctor who has experience removing Essure successfully. You may also want to speak with several doctors before deciding which one is right for you. You can find a list of doctors who have performed Essure removals by state here.
How much does Essure cost?
Under the Affordable Care Act, all FDA-approved methods of birth control, including Essure, must be covered by health insurance plans. That means women with insurance can have Essure inserted at no cost to them.
Unfortunately, having Essure removed is not always covered by insurance and women could pay thousands of dollars out of pocket to have the device removed. Depending on the procedure, Essure removal could cost about $5,000. Hysterectomies are usually covered by insurance, but women without insurance could pay up to $11,000 out of pocket for the surgery.
When did the FDA approve Essure?
Essure was approved by the Food and Drug Administration (FDA) on Nov. 4, 2002. Since then, over 750,000 women have had the Essure device inserted. Thousands of women have reported adverse side effects related to Essure since its approval 14 years ago. Between 2002 and 2015, more than 9,900 adverse events were reported to the FDA regarding Essure.
Who manufactures Essure?
Essure was originally manufactured and marketed by the medical device company Conceptus Inc. In 2013, pharmaceutical giant Bayer bought Conceptus for $1.1 billion.
Has the FDA issued any warnings about Essure?
Yes. The FDA has issued several warnings about Essure since it was approved in 2002. In 2011 and 2012, the FDA required the makers of Essure to update the product label to include nickel sensitivity and pregnancy warnings. In 2013, the FDA required the makers update the Essure label to include the risk of chronic pain and device migration.
Recently, the FDA also announced it would require the makers of Essure to include a black box warning on the label. Black box warnings are the strongest warnings issued by the FDA short of a recall. The agency asked for feedback on what the warning should say and opened it up to the public for 60 days following its announcement back in February 2016.
Has Essure been recalled?
No. Neither the FDA nor the makers of Essure have recalled the device. It is still on the market and available to women seeking permanent birth control.
Are there lawsuits against the manufacturer of Essure?
Yes. The first lawsuit was filed in a Philadelphia court against the manufacturer of Essure in 2014. Despite falling under the FDA’s preemption provision, which says certain medical devices approved under the agency’s premarket approval program are exempt from product liability lawsuits, a Philadelphia judge allowed several lawsuits to proceed in court last March. Since then, hundreds of lawsuits have been filed by women harmed by the controversial birth control device.
I was harmed by Essure. How do I file a lawsuit?
Essure Claims Center Form – click the banner below to see if you qualify:
The lawyers and attorneys at National Injury Help are experienced in claims and cases involving Essure. If you had the Essure device inserted and have experienced adverse effects, call us today for a free case evaluation at 1-800-214-1010. You may be entitled to compensation.
Essure Facts (FAQ) page updated on April 10, 2019.