- Home
- Accident & Injury
- San Diego
- Airplane Accident
- Bicycle Accidents
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Nursing Home Abuse
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- San Diego
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Viagra Skin Cancer Lawsuit
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Birth Injuries
- Commercial Fishing Boat Lawsuit Claims and Settlements
- Cruise Ship Lawsuit Claims and Settlements
- Nursing Home Abuse
- Medical Malpractice

- Sexual Abuse Lawsuits
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
Long-acting, reversible birth control is an increasingly popular method of contraception for women in the United States. More than 10% of women who used contraception 2013 chose IUDs as their preferred method, up from 6% in 2010. The number-one prescribed IUD in the US, called Mirena, came come under scrutiny in recent years after women began reporting serious side effects caused by the device. Learn more about the popular birth control that has potentially dangerous consequences.
What is the Mirena IUD?
Mirena is a reversible, long-acting form of contraception that releases a low dose of hormones over time to prevent pregnancy. Mirena is an intrauterine device, or IUD, made of soft, flexible plastic in the shape of a “T.” It is inserted by a doctor into the uterus where it can last up to five years before it needs to be taken out and replaced if desired.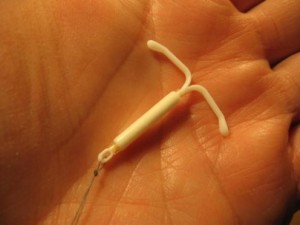
Mirena is the number one IUD in America and has been dubbed the “birth control for busy moms” by its manufacturer. It is recommended for women who have already had children, but has been used by women who do not have children, as well.
How does Mirena work?
Mirena prevents pregnancy by releasing small amounts of the hormone levonorgestrel, a type of synthetic hormone that mimics the naturally occurring hormone progestogen. Mirena does not contain estrogen.
The hormones released by Mirena work to thicken the cervical mucus to prevent sperm from entering the uterus; inhibits sperm from reaching or fertilizing an egg; and thins the lining of the uterus. The makers of Mirena believe the combination of those actions help prevent pregnancy, though the company says there is no single explanation for how Mirena works.
Mirena is inserted into the uterus by a doctor, but contains strings or threads at the end so women can check to make sure it is still in the correct position. When inserted correctly, Mirena is 99% effective at preventing pregnancy.
Who makes Mirena?
Mirena is manufactured by German pharmaceutical company Bayer Healthcare. Bayer is one of the largest pharmaceutical companies in the world, with annual sales of about $51.6 billion in 2015.
When was Mirena approved by the FDA?
Mirena was approved by the Food and Drug Administration (FDA) on Dec. 6, 2000. Mirena is indicated to prevent pregnancy for up to 5 years; it is also approved to treat heavy menstrual bleeding.
How much does Mirena cost?
Mirena is often covered by major insurance plans, which includes the cost of the device, as well as placement and removal by a doctor. If Mirena is not covered by insurance, it can cost upwards of $500 to $900 out of pocket.
Are there generic versions of Mirena?
No, there are no generic equivalents to Mirena.
Are there comparable devices on the market?
There are a few IUDs currently approved by the FDA to prevent pregnancy, including Bayer’s Skyla, Allergan’s Liletta and the copper IUD ParaGard, made by Teva.
Like Mirena, Skyla and Liletta are hormonal IUDs, meaning they contain hormones that are released slowly over time in order to prevent pregnancy. Skyla and Liletta can prevent pregnancy for up to 3 years.
ParaGard, on the other hand, is a non-hormonal birth control. ParaGard is made of copper, which prevents pregnancy without the use of synthetic hormones. ParaGard causes an inflammatory reaction in the uterus that is toxic to sperm.
All of these devices have their pros and cons and may work differently for different women. Other hormonal options for contraception include the pill and NuvaRing.
What are the possible side effects of Mirena?
Mirena has the potential to cause side effects that can range from mild to severe. The following are the most reported side effects associated with Mirena, according to the device’s label:
- Pain, bleeding or dizziness during placement
- Missed menstrual periods (amenorrhea)
- Changes in bleeding, including spotting between periods, longer or shorter periods,
- Benign cysts on the ovary
- Breast tenderness or pain
- Abdominal or pelvic pain
- Headache, migraine
- Back pain
- Acne
- Depression, depressive mood
- Blood clots, stroke
- Device breakage
- Hypersensitivity, including rash, hives, and swelling under the skin
- Increased blood pressure
- Hair loss (alopecia)
- Nausea
- Male-pattern hair growth on the face, chest and back (hirsutism)
- Pelvic inflammatory disease
Other reported side effects include:
- Cramping
- Fatigue
- Vomiting
- Diarrhea
- Bloating
- Weight gain
- Decreased libido
Other side effects
Mirena is often talked about and reviewed on online blogs and forums and many women have reported experiencing certain adverse effects they believe to be caused by the IUD.
Some women have reported that Mirena may have caused weight loss, joint pain and muscle pain and problems with their thyroids.
Women have also reported experiencing problems after having the device removed. Some women have called this the “Mirena crash.” Women have reported post-Mirena side effects like frequent mood swings, sadness, anger, anxiety and depression, tiredness, flu-like symptoms, nausea, bloating and stomach pain and breast tenderness. Women report the side effects can occur for a week or more following the removal of the Mirena device.
Other women have reported that the Mirena device sent them into early menopause so they could not have kids naturally after having the device removed.
It is unclear whether or not Mirena caused the above-mentioned side effects.
What are the possible complications of Mirena?
Mirena has the potential to cause serious, long-term side effects and complications in some women, including migration and perforation, expulsion, sepsis and ectopic pregnancies.
Migration & Perforation
In both clinical trials and real-world practice, Mirena has been shown to perforate, or pierce, the uterus or cervix. In some cases, Mirena can perforate the uterus and migrate into organs in the pelvic or abdominal cavity, including the bladder. In rare cases, the device can perforate the rectum.
Perforation is a potentially serious complication and can cause scarring, infection and damage to other organs. The risk of perforation is about one in 1,000 users.
Studies show that perforation is usually the result of poor placement of the device within the uterus. If the device moves, it may not protect a woman against unwanted pregnancy. A perforated device must be removed and surgery may be required to do so.
Breastfeeding women have a higher risk of perforation because their uterine walls are softer. The Mirena label was updated in 2013 to indicate this risk.
Expulsion
Even if the device doesn’t perforate the uterine wall, it can still be expelled from the correct position and increase the risk for unwanted pregnancy. It is possible for the device to slip slightly from the correct position, called partial expulsion, or it can slip completely out of place, called complete expulsion. A device that is expelled must be removed.
Symptoms of expulsion may include:
- Bleeding
- Pain
- Increased menstrual flow
Sepsis
Sepsis is a potentially life-threatening complication that can occur within the first few days of insertion. Sepsis occurs when the chemicals released by the body into the blood stream to fight infection trigger inflammation, which can lead to tissue damage, organ failure and death.
Symptoms of sepsis may include:
- Fever
- Difficulty breathing
- Abdominal pain
- Unusually fast heart rate
Ectopic pregnancy, pregnancy
The risk of pregnancy while the Mirena device is inserted is about one per 1,000 users, but half of all pregnancies that do occur are ectopic. Ectopic pregnancies occur when the egg implants itself in the fallopian tube instead of the uterus. Ectopic pregnancies require emergency treatment as they can cause internal bleeding, infertility and even death.
Symptoms and ectopic pregnancy include:
- Vaginal bleeding
- Nausea and vomiting with pain
- Lower abdominal pain
- Sharp abdominal cramps
- Dizziness or weakness
Has Mirena been recalled?
Mirena has not been recalled by either the FDA or Bayer.
Has the FDA issued any warnings about Mirena?
The FDA has not issued warnings about Mirena in regard to its safety or efficacy. The FDA has, however, sent warning letters to Bayer regarding Mirena and false and misleading claims the company made during an advertisement.
In 2009, the FDA sent a warning to Bayer about a live consumer-directed program the company made about Mirena. The FDA said the program “overstates the efficacy of Mirena, presents unsubstantiated claims, minimizes the risks of using Mirena, and includes false or misleading presentations regarding Mirena.”
According to the FDA’s warning letter, Bayer claimed Mirena could increase a woman’s level of intimacy and, therefore, make her happier. The FDA said it “is not aware of any evidence that suggests that women using Mirena for birth control experience an increase in reconnection, romance, or intimacy with their partners.”
Bayer also claimed Mirena could make women “look and feel great,” and falsely stated the device required no monthly routines despite the fact that women are advised to have their IUD checked 4 to 12 weeks after initial insertion and once a year thereafter.
Have lawsuits been filed against the maker of Mirena?
Women who were injured by the Mirena IUD have begun filing lawsuits against Bayer Healthcare saying the company failed to warn them about potential risks of the device.
Cases have been filed in federal court and are consolidated in a multidistrict litigation (MDL) in the U.S. District Court for the Southern District of New York.
U.S. District Judge Cathy Seibel is presiding over the cases which have now reached over 1,300.
I suffered harm as a result of the Mirena IUD. Can I file a lawsuit?
If you or a loved one was injured by the Mirena IUD, you may be entitled to compensation but you need to act now as cases are currently pending in U.S. federal court.
The Mirena lawyers and attorneys at National Injury Help can help you take on the pharmaceutical companies and seek the compensation you deserve.
Call National Injury Help today to speak with a member of our legal team. We can answer your questions and help you determine if you have a case. Call us at 1-800-214-1010 for a free case evaluation or use the form on the right-hand side of your screen.





