- Home
- Accident & Injury
- Locations
- Arizona
- Avondale
- Avondale Personal Injury Lawyer
- Avondale Car Accident Lawyer
- Avondale Truck Accident Lawyer
- Avondale Bicycle Accident Lawyer
- Avondale Pedestrian Accident Lawyer
- Avondale Motorcycle Accident Lawyer
- Avondale Rideshare Accident Lawyer
- Avondale Wrongful Death Attorney
- Avondale Dog Bite Lawyer
- Avondale Brain Injury Attorney
- Buckeye
- Buckeye Personal Injury Lawyer
- Buckeye Car Accident Lawyer
- Buckeye Truck Accident Lawyer
- Buckeye Motorcycle Accident Lawyer
- Buckeye Bicycle Accident Lawyer
- Buckeye Pedestrian Accident Lawyer
- Buckeye Rideshare Accident Lawyer
- Buckeye Wrongful Death Attorney
- Buckeye Dog Bite Lawyer
- Buckeye Brain Injury Attorney
- Casa Grande
- Casa Grande Personal Injury Lawyer
- Casa Grande Car Accident Lawyer
- Casa Grande Truck Accident Lawyer
- Casa Grande Motorcycle Accident Lawyer
- Casa Grande Bicycle Accident Lawyer
- Casa Grande Pedestrian Accident Lawyer
- Casa Grande Rideshare Accident Lawyer
- Casa Grande Wrongful Death Attorney
- Casa Grande Brain Injury Attorney
- Casa Grande Dog Bite Lawyer
- Chandler
- Chandler Personal Injury Lawyer
- Chandler Car Accident Lawyer
- Chandler Truck Accident Lawyer
- Chandler Motorcycle Accident Lawyer
- Chandler Bicycle Accident Lawyer
- Chandler Rideshare Accident Lawyer
- Chandler Pedestrian Accident Lawyer
- Chandler Wrongful Death Attorney
- Chandler Dog Bite Lawyer
- Chandler Brain Injury Attorney
- Florence
- Florence Personal Injury Lawyer
- Florence Car Accident Lawyer
- Florence Truck Accident Lawyer
- Florence Motorcycle Accident Lawyer
- Florence Bicycle Accident Lawyer
- Florence Pedestrian Accident Lawyer
- Florence Rideshare Accident Lawyer
- Florence Dog Bite Accident Lawyer
- Florence Brain Injury Attorney
- Florence Wrongful Death Attorney
- Gilbert
- Gilbert Personal Injury Lawyer
- Gilbert Car Accident Lawyer
- Gilbert Truck Accident Lawyer
- Gilbert Motorcycle Accident Lawyer
- Gilbert Bicycle Accident Lawyer
- Gilbert Pedestrian Accident Lawyer
- Gilbert Rideshare Accident Lawyer
- Gilbert Wrongful Death Attorney
- Gilbert Dog Bite Lawyer
- Gilbert Brain Injury Attorney
- Glendale
- Glendale Personal Injury Lawyer
- Glendale Car Accident Lawyer
- Glendale Truck Accident Lawyer
- Glendale Motorcycle Accident Lawyer
- Glendale Bicycle Accident Lawyer
- Glendale Pedestrian Accident Lawyer
- Glendale Rideshare Accident Lawyer
- Glendale Wrongful Death Attorney
- Glendale Dog Bite Lawyer
- Glendale Brain Injury Attorney
- Goodyear
- Goodyear Personal Injury Lawyer
- Goodyear Car Accident Lawyer
- Goodyear Truck Accident Lawyer
- Goodyear Motorcycle Accident Lawyer
- Goodyear Bicycle Accident Lawyer
- Goodyear Rideshare Accident Lawyer
- Goodyear Pedestrian Accident Lawyer
- Goodyear Wrongful Death Attorney
- Goodyear Brain Injury Attorney
- Goodyear Dog Bite Lawyer
- Mesa
- Peoria
- Phoenix
- Phoenix Personal Injury Lawyer
- Phoenix Car Accident Lawyer
- Phoenix Truck Accident Lawyer
- Phoenix Motorcycle Accident Lawyer
- Phoenix Bicycle Accident Lawyer
- Phoenix Pedestrian Accident Lawyer
- Phoenix Rideshare Accident Lawyer
- Phoenix Wrongful Death Attorney
- Phoenix Dog Bite Lawyer
- Phoenix Brain Injury Attorney
- San Diego
- Airplane Accident
- Brain Injury
- Boating Accident
- Car Accident
- Construction Accident
- Dog Bite
- Motorcycle Accident
- Pedestrian Accident
- Rideshare Accident
- Slip and Fall
- Spinal Cord Injury
- Uber Accident
- Truck Accident
- Train Accident
- Workers’ Compensation
- Wrongful Death
- Carlsbad
- Coronado
- EI Cajon and La Mesa
- Escondido and San Marcos
- Fallbrook
- Hillcrest
- Lakeside
- Lemon Grove
- Oceanside
- Santee
- Pacific Beach and Mission Beach
- Poway
- Scottsdale
- Scottsdale Personal Injury Lawyer
- Scottsdale Car Accident Lawyer
- Scottsdale Truck Accident Lawyer
- Scottsdale Motorcycle Accident Lawyer
- Scottsdale Bicycle Accident Lawyer
- Scottsdale Pedestrian Accident Lawyer
- Scottsdale Rideshare Accident Lawyer
- Scottsdale Wrongful Death Attorney
- Scottsdale Brain Injury Attorney
- Scottsdale Dog Bite Lawyer
- Surprise
- Surprise Personal Injury Lawyer
- Surprise Car Accident Lawyer
- Surprise Truck Accident Lawyer
- Surprise Motorcycle Accident Lawyer
- Surprise Bicycle Accident Lawyer
- Surprise Pedestrian Accident Lawyer
- Surprise Rideshare Accident Lawyer
- Surprise Wrongful Death Attorney
- Surprise Dog Bite Lawyer
- Surprise Brain Injury Attorney
- Tempe
- Tucson
- Yuma
- Defective Drugs
- Abilify Lawsuits
- Actos
- Benicar lawsuits, claims and Settlements
- Bravelle Lawsuit Claims Settlements
- Byetta
- Celexa
- Cipro Lawsuit Settlements
- Concerta Lawsuit
- Effexor
- Levaquin Lawsuit Settlements
- GranuFlo
- Invokana Lawsuit Claims & Settlements
- Fosamax
- Janumet
- Januvia
- Lexapro
- Lipitor
- Omontys
- Onglyza Lawsuit Claims Settlements
- Plavix
- Pradaxa
- Propecia
- Proton Pump Inhibitor PPIs lawsuit
- Risperdal
- Taxotere hair loss lawsuit
- Xarelto Lawsuit
- Zofran
- Proscar
- Prozac
- SSRI Birth Defects
- Topamax
- Tylenol
- FAQs About Viagra
- Victoza
- Z-Pak
- Zofran Claims Canada
- Zoloft
- custom
- Defective Products
- Artelon Spacer
- Atrium C-Qur Hernia Mesh Lawsuit
- Baby Food Autism Lawsuit
- Bair Hugger Infection Lawsuits
- Bard Sepramesh IP Composite Mesh Lawsuits
- Biomet Comprehensive Shoulder Implant Lawsuit
- Da Vinci Robotic Surgery
- Riata Leads
- Essure Birth Control Lawsuit
- Filshie clips lawsuits
- Hip Implant Lawsuits
- IVC Filter Lawsuits
- Medtronic Bone Graft
- Mirena IUD Lawsuit Claims & Settlements
- Monsanto Roundup Cancer Lawsuit
- Power Morcellator Lawsuit
- Talcum Powder Lawsuits
- FAQs About Zimmer Persona Knee Implants
- Military PFAS Contamination Lawsuit
- Spinal Cord Stimulator Lawsuit Claims
- Stryker Hip Replacement Lawsuit Claims & Settlements
- Stryker V40 Recall Lawsuit Claims & Settlements
- Social Media Addiction Lawsuit
- Suboxone Tooth Decay Lawsuit
- Textured breast implant cancer lawsuits
- Vaginal Mesh
- Watchman Stroke Device Lawsuit
- Wright Hip Replacement Lawsuit, Claims & Settlements
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Demanda de DuPont & Chemours Teflon
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
- Other Case Types
- Sexual Abuse Lawsuits
- Clergy Priest Sexual Abuse Lawyers, California Church Crimes
- List: Every abusive Catholic Church priest, clergy member named in every state in the past year.
- Clergy Priest Sexual Abuse Lawyers, New York Church Crimes Attorney
- Clergy Priest Sexual Abuse Lawyers, New Jersey Church Crimes
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- Massage Envy Lawsuit Sexual Assault Claims
- An Insight into the Sexual Abuse within Boy Scouts of America
- Clergy Priest Sexual Abuse Lawyers, Pennsylvania Church Crimes
- An Insight into the Sexual Abuse within Boy Scouts of America
- How to Help a Child Who Has Been Abused
- Where to Find Names of Clergy Accused of Sex Abuse
- Musicians Who Have Been Charged With Sexual Abuse
- Birth Injuries
- Commercial Fishing Boat Lawsuit Claims and Settlements
- Cruise Ship Lawsuit Claims and Settlements
- Nursing Home Abuse
- Medical Malpractice
- Sexual Abuse Lawsuits
- Blog
- Contact Us
- En Español
- abogados para usted
- Asbesto en Casa
- Blog En Espanol
- Noticias de accidentes
- Demanda de Abilify
- Demanda de Actos
- Demanda de Implante de Hombro Comprehensive de Biomet
- Resolución de Reclamaciones de demanda de Bravelle
- Demanda de Celexa
- Demanda de Parálisis Cerebral, Acuerdos de Demanda
- Acuerdo de Demanda de Cipro
- Demanda de Concerta
- Demanda contra el Implante Anticonceptivo Essure
- Demanda de Malla Flexible de Ethicon
- Demanda de Malla para Reparación de Hernias C-Qur de Atrium
- Acuerdos de demanda de Invokana
- Acuerdo de demanda de Levaquin
- Demandas contra el filtro IVC
- Demanda de Mesotelioma
- Demanda Monsanto Roundup cancer
- Demanda de Inhibidores de la Bomba de Protones (PPI)
- Demanda de Pradaxa
- Demanda de Propecia
- Demanda contra de Risperdal
- Demanda de Taxotere por pérdida de cabello
- Demanda de Tylenol
- Demanda de Viagra por Cáncer de Piel
- Demanda contra Xarelto
- Demanda de Zofran por Defectos Congénitos
Call 24 Hours - Toll Free 1 (800) 214-1010
Did you suffer serious complications or side effects after having a Watchman stroke device implanted?
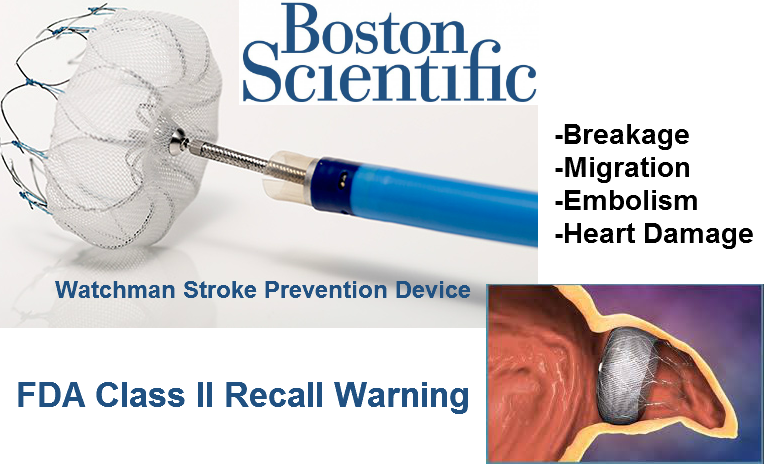
The Food and Drug Administration recalled some Watchman Left Atrial Appendage Closure Devices made by Boston Scientific in late 2015, raising concerns about the safety of this stroke prevention device.
Potential Watchman Stroke Device Lawsuits forming
Lawsuits may be forming against Boston Scientific, the maker of the Watchman LAA Closure Device, because of complications allegedly suffered by patients who had the device implanted.
If you or someone you love was injured as a result of having the Watchman device implanted, you may be entitled to compensation. Call the experienced lawyers at National Injury Help today for a free consultation to see if you qualify for a Watchman stroke device claims lawsuit. There may be substantial cash awards from lawsuit settlements for this Boston Scientific device.
Call 1-800-214-1010 at any time for a free case evaluation or use the form on the right hand side of the screen.
Watchman Device Complications & Problems
The Watchman Left Atrial Appendage (LAA) Closure Device is a cage-like device used to prevent blood clots from traveling to the brain and causing a stroke in people with a heart condition called non-valvular atrial fibrillation, or AF. The Watchman device is meant to eventually replace long-term anticoagulation therapy in these patients.
The Watchman is surgically implanted in the heart’s left atrium appendage, or LAA, a small pouch that connects and drains into the left atrium and where the majority of blood clots that cause strokes in AF patients originate. The Watchman is permanently implanted in this pouch to create a seal and prevent blood clots from traveling to the brain.
The Food and Drug Administration approved the Watchman in March 2015 using an expedited review process under the agency’s Pre-Market Approval program. But merely six months after its approval, the Watchman’s manufacturer, Boston Scientific, initiated a recall of nearly 30,000 units due to potential complications during the implantation procedure.
The FDA classified the September 2015 recall as a class II, meaning temporary or reversible adverse health effects were possible.
The Watchman device has been linked to serious adverse health consequences, including migration of the device, fracture of the device, damage to heart valves and the potential need for additional surgeries due to complications.
Individuals harmed by the Watchman LAA Closure Device may be able to seek settlements in the form of a Watchman Lawsuit.
Watchman Class II Recall
On September 15, 2015, Boston Scientific initiated a class II recall of its Watchman device and access sheath, which helps guide the Watchman into the heart. Nearly 30,000 units of the Watchman and 30,000 units of the access sheath were recalled in over 50 countries worldwide.
The FDA-determined cause of the recall was “use error.” Boston Scientific said it initiated the recall because blood leakage could occur if implanting surgeons tightened the hemostasis valve with the dilator in place. The company subsequently updated the Watchman’s Direction for Use to give further guidance on correct implantation of the device.
The class II recall is still ongoing as of September 2016; however, Watchman devices not affected by the recall remain on the market and are still being used today.
Watchman Stroke Device Side Effects & Adverse Events
The Watchman device has only been on the market for a short time, so all of the potential adverse events associated with the device are not yet known.
Some of these adverse events occur because of the device itself, while others can occur during the implantation procedure.
Known serious adverse events associated with the Watchman device include:
- Device embolism
- Device fracture
- Inability to reposition or retrieve device
- Need for surgical removal
- Valvular damage
- Misplacement of the device
- Cardiac perforation
- Major bleeding
- Death
Other adverse events, which also may be serious that are associated with the device, include:
- Allergic reaction
- Altered mental status
- Anemia
- Bruising
- Hematoma
- Chest pain/discomfort
- Confusion post procedure
- Congestive heart failure
- Cranial bleed
- Deep vein thrombosis
- Fever
- Groin pain
- Renal failure
- Pulmonary edema
Watchman Implant Procedure
The Watchman device is surgically implanted inside the patient, typically under general anesthesia. The procedure lasts on average about one hour and patients usually spend the next 24 hours in the hospital.
Surgeons generally implant the Watchman device through a small incision in the groin area. After determining the correct size of device, surgeons use the access sheath to guide the device into the left atrial appendage in the heart where it is deployed to capture blood clots.
The device is intended to remain inside a patient’s heart permanently. The cost of the Watchman device is covered by Medicare and Medicaid as of February 8, 2016.
Studies highlight complications associated with stroke device
The Watchman LAA device has been linked to complications that can arise during the implantation procedure. Boston Scientific estimates the complication rate of its Watchman device is about 4%, and acknowledges there is a “learning curve” associated with implantation.
This learning curve was discovered during Boston Scientific’s own clinical trials, which found a greater percentage of patients experienced “safety events” with less experienced implanting surgeons than those with more experienced surgeons.
During the company’s PROTECT AF trial, 9.9% of patients experienced a safety event during the first half of the trial compared to 4.8% during the second half. Researchers established the learning curve during this trial was 5.1%.
American Heart Association Study 2016
In July 2016, the American Heart Association published a study in the journal Circulation: Cardiovascular Quality and Outcomes indicating devices like the Watchman were associated with “high rates of procedure-related harms.”
Citing numerous observational studies, the AHA said there is “moderate-strength evidence of serious harms” associated with devices like the Watchman.
The study also concluded there is little evidence showing the device is “non-inferior” to long-term anticoagulation therapy – directly contradicting the results of Boston Scientific’s PREVAIL study, which found the Watchman to be “noninferior” to long-term treatment with warfarin.
Watchman July 2015 Study
A study published by researchers from Belgium and Greece in the journal Catheterization & Cardiovascular Interventions in July 2015 found certain complications with the Watchman could occur not only shortly after the implantation procedure, but also later on down the line.
Researchers analyzed different studies reporting cases of device embolization, or movement of the device from its original location in the heart’s LAA to other areas of the body, and found the Watchman had moved into the aorta, the left ventricle or the left atrial cavity. Movement of the device into the left ventricle was associated with a higher rate of surgical retrieval.
The cases indicated most of the embolizations were acute, or occurring shortly after implantation. This may suggest complications arose because of implantation errors during the initial surgery. However, researchers also found case of late embolization. This could suggest those complications did not arise from implantation errors.
Researchers highlighted the importance of their study, saying “… little is known about the outcome of patients with device emobolization, the anatomical location of [the] embolized device and the methods for device retrieval.”
Studies have linked the device to potential complications, such as movement of the device, or device embolization, and 30,000 units of the device were recalled due to “use error” in September 2015.
The National Injury Help is currently investigating cases for a potential lawsuit against Boston Scientific. If you or someone you love suffered complications like device embolization after being implanted with a Watchman device, you may be entitled to financial compensation.
Call National Injury Help today to speak with a member of our legal team. We can answer your questions and help you determine if your case qualifies for a possible Watchman Lawsuit.
Boston Scientific Watchman Stroke Device Lawsuit Claims & Settlements page updated on 4/10/2019