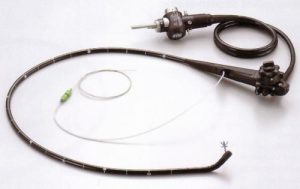
Nov. 18, 2016 – San Diego, CA — A U.S. medical supplies company unveiled a brand new power morcellator this week after gaining U.S. Food and Drug Administration approval early last month.
It is the first power morcellator to be approved using new containment bag technology and the second approved since the FDA warned about the risk of spreading cancer when using morcellators to remove uterine fibroids.
 First-of-its-kind morcellator
First-of-its-kind morcellator
Olympus Medical announced the arrival of its first-of-its-kind power morcellator at the American Association of Gynecologic Laparoscopists Annual Meeting on Nov. 15.
The Olympus Contained Tissue Extraction System is comprised of two FDA-approved medical devices in one: the PK Morcellator and PneumoLiner containment bag. The morcellator is used to chop up tissue during minimally invasive (laparoscopic) surgeries, while the bag contains the chopped up tissue and keeps the particles from spreading throughout the body.
The PneumoLiner is manufactured by Advanced Surgical Concepts and was first approved by the FDA in April 2016. It was designed in response to a 2014 FDA warning about the risk of power morcellators spreading undetected cancers in women undergoing hysterectomies or myomectomies to remove uterine fibroids.
The FDA estimates that 30% to 40% of the nearly 600,000 hysterectomies performed each year in the U.S. are to remove uterine fibroids and power morcellation was used in roughly 60,000 procedures each year before the FDA’s 2014 warning.
The use of power morcellators rose dramatically in the last two decades, increasing from 30% of hysterectomies in 2002 to about 63% in 2012, according to a press release issued by Olympus Medical Nov. 16.
Their use dropped rapidly following the FDA’s warning, which cautioned physicians about using power morcellators to chop up uterine fibroids during minimally invasive hysterectomies and myomectomies. The morcellators, the warning indicated, could spread undetected uterine or ovarian cancer during the procedure, dramatically reducing a woman’s chance for long-term survival.
About 1 in 350 women has undetected cancer before undergoing surgery to remove uterine fibroids, according to the FDA. The agency admits there is no reliable method for predicting whether a woman with fibroids may have uterine cancer and discourages the use of power morcellators during these procedures.
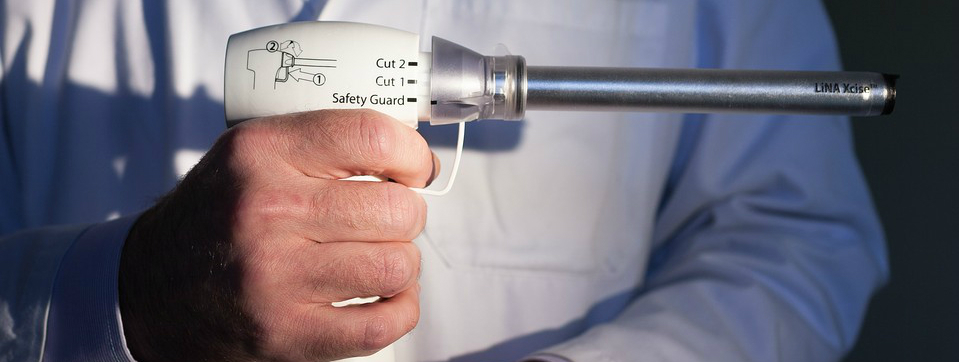 Not proven to reduce spread of cancer
Not proven to reduce spread of cancer
In April 2016, the FDA approved the first containment system for use with certain power morcellators in select patients. The PneumoLiner is intended to contain morcellated tissue and to possibly prevent the spread of undetected cancers, but the FDA has not changed its position regarding the risks of power morcellation.
“We are continuing to warn against the use of power morcellators for the vast majority of women undergoing removal of the uterus or uterine fibroids,” Chief Scientist at the FDA’s Center for Devices and Radiological Health Dr. William Maisel said in a press release issued back in April. “This new device does not change our position on the risks associated with power morcellation.”
The FDA also made clear that the PneumoLiner’s ability to reduce the spread of potentially cancerous tissue during power morcellation has not been proven. The device is only approved for use in a limited patient population, including women without uterine fibroids undergoing a hysterectomy and some pre-menopausal women with fibroids who want to maintain their fertility.
Olympus’ new power morcellation system combines its PK Morcellator with the PneumoLiner, but does not appear to have restrictions on patient population, according to its approval letter. It is unclear whether the restrictions placed on PneumoLiner extend to the new combination system, but the fact that the containment bag’s ability to reduce the spread of cancer remains unproven has not changed.
Approved without rigorous testing
The FDA approved Olympus’ new power morcellation system in early October through its 510(k) process. This means the new system was similar enough to other medical devices already on the market for the company to skip the rigorous clinical tests required of new, class III medical devices.
Despite the Olympus system being a “first-of-its-kind” device, its manufacturer was allowed to circumvent the stringent clinical testing required of other new devices.
Black Box Warnings
Olympus’ brand new power morcellation system carries an FDA-required black box warning to alert physicians about the risk of spreading undetected cancer during surgeries to remove uterine fibroids.
The black box warning can be found on both the PneumoLiner and PK Morcellator labels, along with other device-specific contraindications.
The PneumoLiner has required a black box warning since it was approved by the FDA in April 2016:

A snapshot of the black box warning and contraindications listed on the PneumoLiner’s label, taken from the Olympus website.
The PK Morcellator also carries a black box warning that was required upon approval:

A look at the black box warning and contraindications on the PK Morcellator label, taken from the Olympus website.
Physician testing required, but Olympus overseeing
In addition to a black box warning, the FDA mandated that all physicians undergo training and education before using the new Olympus system.
“Surgeons who wish to use the containment system will be required to complete a formal training protocol before use,” Olympus wrote in its Nov. 16 press release.
The mandated training program was developed by Olympus and Advanced Surgical Concepts, the manufacturer of the PneumoLiner, and will be executed by Olympus, according to the company’s release.
“[The training program] will be implemented by Olympus with surgeon validation to ensure that healthcare professionals use the system safely and effectively,” the press release continued.
Without FDA oversight, can a company whose goal is to maximize profits and whose obligation lies with its stakeholders responsibly employ the physician training program? Or will its goal of earning profits outweigh the goal of adequately training physicians?
 Olympus settles claims it bribed doctors to increase sales
Olympus settles claims it bribed doctors to increase sales
Olympus Medical was caught in a media firestorm after deadly superbug outbreaks in Europe and the U.S. were traced back to the company’s duodenoscope medical devices.
In March 2016, Olympus settled allegations it paid illegal kickbacks to doctors in the U.S. and Central and South America in order to maximize sales of medical devices, including duodenoscopes, according to a press release by the U.S. Dept. of Justice.
The company paid out more than $623 million to the U.S. government to settle criminal and civil charges, the largest total amount paid by a medical device company in U.S. history.
The criminal complaint against Olympus claimed the company earned more than $600 million in sales by giving doctors and hospitals kickbacks to buy its products. These included consulting payments, foreign travel, lavish meals, millions of dollars in grants, and free medical equipment, according to the release.
The company was accused of illegal activities such as withholding a $50,000 research grant until a hospital signed a deal to purchase Olympus equipment, and giving a hospital $5,000 in grant money to close a $750,000 sale, among other allegations.
Olympus reportedly admitted to these charges, but the accusations didn’t stop there.
Olympus’ Latin American subsidiary was accused of implementing a five-year plan to increase sales in Central and South America by providing doctors with cash, money transfers, personal grants, personal travel, and free or heavily discounted medical equipment. To do this, the company reportedly set up “training centers” to educate and train doctors, but used those centers to provide benefits to pre-selected physicians, the Dept. of Justice press release states.
“Olympus Latin America admitted to bribing publicly employed health care providers and hospital officials across Central and South America so that it could illegally win business and sell its products,” Principal Deputy Assistant Attorney General David Bitkower said in the release.
Olympus leadership acknowledged the company’s responsibility for its past conduct and said it did not represent the values of Olympus or its employees in a press release issued to its partners and stakeholders.
Can a company who has already admitted to setting up fake training centers to illegally maximize its sales and profits be trusted to set up training programs for doctors using its new power morcellator system? Or will this be another tool used by Olympus to push sales of its newest device?
Power Morcellator Lawsuits
Power Morcellator Cancer Lawsuits continue to mount against the manufacturers of these devices. If you or someone you love was diagnosed with cancer after undergoing a hysterectomy with a power morcellator, you may be entitled to compensation. With power morcellator cases already settling out of court, it is important that you act now.
The experienced Power Morcellator Attorneys at National Injury Attorneys, LLC are ready to fight for you and your rights. Call us at 1-800-214-1010 to speak with a member of our legal team and see if you qualify for a Power Morcellator Cancer Lawsuit. We are available at any time day or night, and the initial consultation is absolutely free. You can also use the form on the right-hand side of your screen.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- When Pet Owners Fail to Control Aggressive Dogs: Legal Options for Bite Victims in California
- Rideshare Driver or Passenger? Legal Steps to Take After a California Uber or Lyft Accident
- Crosswalk Accidents: When California Drivers Fail to Yield and Pedestrians Pay the Price
- Parents, Teens, and Texting: Addressing the Dangers of Distracted Driving Among Young Drivers in California
- California Rideshare Accidents Involving Minors: What Parents Need to Understand