June 8, 2018 – San Diego, CA. Hip replacement surgery is a viable option for people who suffer from limited mobility due to hip pain. However many may face a side effect know as metallosis from the hip replacement or a similar side effect called cobalt poisoning.
There are numerous types of implants on the market, but some may be safer than others. Thousands of patients learned that the hard way after suffering severe, painful complications due to faulty devices. This article will help familiarize you with hip replacement surgery, the different types of hip implants, and which hip implants you may want to think twice about getting.
The basis – What are hip implants?
The hip is the second largest weight-bearing joint in the body after the knee. Hip implants replace the parts of the hip joint that may become damaged over time due to various conditions or diseases. Hip implants help people with pain and limited mobility due to a damaged hip joint regain their quality of life. 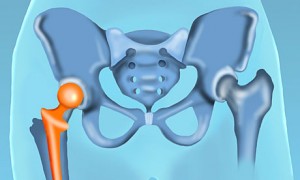
When talking about hip implants, it’s important to understand the anatomy of the hip joint. The hip is a ball-and-socket joint. A rounded protrusion at the top of the femur (the thighbone) forms the ball and a cup-shaped area in the pelvis, called the acetabulum, forms the socket. The ball fits into the socket and is held there with the help of ligaments and muscles. Thin tissue, called the synovial membrane, surrounds the hip joint inside the socket and provides lubrication for smooth movement.
Hip implants are designed to replace the ball and socket of the hip joint when they become damaged. Hip implants are made of several different components:
- Femoral stem – A metal stem inserted into the thighbone that holds the ball joint in place. The femoral stem can be cemented into the bone or “press fit” to allow new bone growth to hold it in place.
- Femoral head – A metal or ceramic ball placed on the upper part of the femoral stem. The femoral head replaces the damaged femoral head that was removed.
- Acetabular component – A metal socket that replaces the damaged cartilage removed from the acetabulum. It is sometimes held in place using screws or cement.
- Liner – A plastic, metal or ceramic spacer inserted between the ball and socket to allow for a smooth gliding surface.
What is metallosis from a hip replacement?
All hip implants, regardless of the material, can be worn down due to everyday movement. Typically, hip implants last about 10 to 20 years, but some MoM implants have been shown to fail much earlier.
One study released in the BMJ in 2012 indicated an average failure rate at seven years of 13.6 percent for MoM implants compared to 3.3 percent to 4.9 percent for implants made from other materials. This means the need for earlier revisions is higher in MoM implants.
As implants wear down, particles from the implant material are released and can settle in and around the joint. Particles from MoM implants, namely chromium and cobalt ions, can damage surrounding tissues and bone and enter the blood stream. This can lead to complications known as Adverse Local Tissue Reaction (ATLR) and Metallosis (metal toxicity).
The tissue damage associated with ATLR can cause necrosis – the death of most or all of the cells in the tissue – as well as the accumulation of lymph cells.
Metallosis is caused by the buildup of metal debris in the soft tissues of the body, even those outside of the joint area. It is estimated that five percent of patients who were implanted with any type of metal joint developed metallosis over the past 40 years.
Both ATLR and metallosis can lead to pain, implant loosening and failure and the need for revision surgery.
Some case reports have also shown patients with MoM implants could develop symptoms or illnesses elsewhere in the body, including:
- Skin rashes
- Cardiomyopathy (disease of the heart muscle)
- Hearing or vision impairments
- Psychological changes (depression or cognitive impairment)
- Impaired kidney function
- Thyroid dysfunction (neck discomfort, fatigue, weight gain, feeling cold)
The FDA recommended doctors monitor patients who experienced pain or swelling near the hip, a change in walking ability or noise from hip joint after three months of receiving their MoM implant.
What is cobalt or chromium poisoning from hip implants?
Studies performed and gathered by the National Center for Biotechnology Information published a comprehensive article on January 24, 2017 entitled: Neuropsychiatric symptoms following metal-on-metal implant failure with cobalt and chromium toxicity. Its methods involved evaluating ten cases of patients (average age 60) where they looked at neuropsychiatric morbidity following metal-on-metal hip implant failure and revision.
Implants were ASR total hip replacement (acetabular implant, taper sleeve adaptor and unipolar femoral implants) performed between 2005 and 2009. This case series describes, for the first time, neuropsychiatric complications after revision where there has been cobalt and chromium toxicity.
In the study the results presented (directly from the ncbi.nih.gov site) are as follows: 
“Pre-revision surgery, nine patients had toxic levels of chromium and cobalt (mean level chromium 338 nmol/l, mean cobalt 669.4 nmol/l). Depression assessment showed 9 of 9 respondents fulfilled the BDI criteria for depression and 3 of these were being treated. 7 of 9 patients showing short term memory deficit with mean mini mental state examination score of 24.2. The normal population mean MMSE for this group would be expected to be 28 with <25 indicating possible dementia.”
Conclusions – “We found neurocognitive and depressive deficits after cobalt and chromium metallosis following MoM implant failure. Larger studies of neurocognitive effects are indicated in this group. There may be implications for public health.
Keywords: Hip implant, Cobalt, Chromium, Depression, Cognitive, Neuropsychiatric, Metal-on-metal”
What hip replacement brands cause metallosis?
There a numerous manufacturers currently marketing hip implants in the United States. Some of these manufacturers have marketed problematic devices that were later recalled. Those manufacturers include:
- DePuy Orthopaedics
- Zimmer Holdings
- Stryker Orthopaedics
- Biomet
- Wright Medical Technology
- Smith & Nephew
- OMNI
Are there any lawsuits related to Metallosis hip replacements?
Several hip implant manufacturers are facing lawsuits from patients who say they suffered complications as a result of their devices.
Currently, Johnson & Johnson subsidiary DePuy faces thousands of lawsuits for its Pinnacle and ASR hip implant systems. These lawsuits have been consolidated into multidistrict litigation (MDL) in federal court. Roughly 8,350 lawsuits are pending in the U.S. District Court for the Northern District of Texas against DePuy’s Pinnacle implant; about 2,360 lawsuits are pending in the U.S. District Court for the Northern District of Ohio against DePuy’s ASR hip implant.
Wright Medical Technology Inc. faces roughly 550 lawsuits pending in the U.S. District Court for the Northern District of Georgia against its Conserve hip implant.
Biomet faces nearly 1,240 lawsuits currently pending in the U.S. District Court for the Northern District of Indiana against its M2a Magnum hip implant.
About 1,600 lawsuits against Stryker and its Rejuvenate and ABG II hip implants are pending in the U.S. District Court for the District of Minnesota.
Zimmer faces over 400 lawsuits pending in the U.S. District Court for the District of New Jersey for its Durom hip cup.
Current hip replacement lawsuits are active and ready to settle.
As stated above, several different companies have been on the receiving end of complaints – both verbal and legal in nature – because of the alleged failings of their products. The National Injury Attorneys, LLC, is a team of defective hip implant device lawyers that has been standing up for the rights of clients all over the United States who suddenly found themselves suffering for reasons they could never have foreseen. Examples of companies that have been involved in these cases include:
- Stryker
- Wright
- Smith and Nephew
- DePuy
- Biomet
- Sulzer
There have been others, but consumers clearly have no ability to evaluate the quality of a hip implant device before it’s put into their bodies, and doctors should be able to rely on reasonable warranties and the like before performing surgeries.
If you or someone you love has had hip surgery and you’re suffering because of a defective device, you need to take action to protect your legal rights. Contact the National Injury Attorneys, LLC, today to schedule a free initial consultation so that you can obtain the help you need recovering the compensation that you deserve.
Blog post: Metallosis hip replacement -cobalt poisoning hip replacement symptoms published on June 8, 2018.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- When Pet Owners Fail to Control Aggressive Dogs: Legal Options for Bite Victims in California
- Rideshare Driver or Passenger? Legal Steps to Take After a California Uber or Lyft Accident
- Crosswalk Accidents: When California Drivers Fail to Yield and Pedestrians Pay the Price
- Parents, Teens, and Texting: Addressing the Dangers of Distracted Driving Among Young Drivers in California
- California Rideshare Accidents Involving Minors: What Parents Need to Understand






