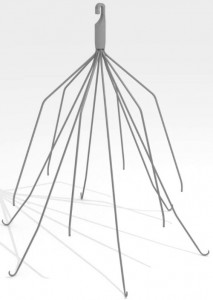
Picture of Bard IVC Filter
August 17, 2016 – San Diego, CA. IVC Filters are used to help prevent blood clots from moving through the body by acting like a type of trap for a predetermined size of blood clot. They are inserted via a surgical process into the inferior vena cava (a large blood vein that extends from the lower torso up to the heart).
The risk of this device is called migration, where the legs of the filter can actually break off and travel through the body. This in turn leads to the possible risk of pulmonary embolism or the device can migrate into the lungs or heart causing potential life threatening situations.
What IVC Filter Brands break?
There are 4 brands of these IVC filters that are prone to breakage and they include:
Bard Peripheral Vascular
- Recovery vena cava filter (discontinued)
- G2 vena cava filter
- G2 X vena cava filter
- Eclipse vena cava filter
Boston Scientific products include:
- Titanium Greenfield filter (TGF)
- Percutaneous stainless steel Greenfield filter (12F SGF)
- Stainless steel Greenfield filter (SGF) (Kimray-Greenfield filter)
B Braun Medical products include:
- Vena Tech LGM vena cava filter
- Vena Tech LP vena cava filter
Cook Medical products include:
- Gianturco-Roehm Bird’s Nest filter (BNF)
- Gunther Tulip vena cava filter
- Celect vena cava filter
FDA Issues High Level Warning for IVC Filters.
Early in 2005, the FDA started receiving 921 device adverse event reports involving IVC filters, they include:
- 328 involved device migration.
- 146 involved embolizations (detachment of device components).
- 70 involved perforation of the IVC.
- 56 involved filter fracture.
Some of these events led to adverse clinical outcomes in patients. These types of events may be related to a retrievable filter remaining in the body for long periods of time, beyond the time when the risk of pulmonary embolism (PE) has subsided.
The FDA is concerned that these retrievable IVC filters, intended for short-term placement, are not always removed once a patient’s risk for PE subsides. Known long term risks associated with IVC filters include but are not limited to lower limb deep vein thrombosis (DVT), filter fracture, filter migration, filter embolization and IVC perforation.
We can help you file an IVC Filter lawsuit claim.
If you or someone you know has had an IVC Filter and has had any side effect or have died, contact us today for a free case review as a class action lawsuit may take place with substantial cash claims awards from settlements.
Note: The information provided in this article is based on reports from publicly available sources, including news outlets, police reports, and eyewitness accounts. National Injury Help has not independently verified all details of the reported incident. If you find any inaccurate or outdated information, please contact us, and we will review and update the content as appropriate. The photo used in this post is for illustrative purposes only and does not depict the actual scene of the incident.
Disclaimer: The content of this article is intended for informational purposes only and does not constitute legal advice or establish an attorney-client relationship with National Injury Help. For legal assistance specific to your case, we encourage you to contact a qualified attorney.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- Scottsdale, AZ – Choque múltiple en la Ruta 101 deja heridas graves
- Phoenix, AZ – Hombre muere tras ser atropellado en accidente peatonal en Buckeye Rd
- Scottsdale, AZ – Multi-Vehicle Crash on Loop 101 Leaves Two Seriously Hurt
- Phoenix, AZ – Man Fatally Struck in Pedestrian Crash on Buckeye Rd
- Los Beneficios de Mantener un Estilo de Vida Saludable







