 Jan. 3, 2017 – San Diego, CA — When the U.S. Food and Drug Administration convened an advisory panel meeting in September 2015 to discuss the safety and effectiveness of Essure, the permanent birth control device had already been on the market for 13 years.
Jan. 3, 2017 – San Diego, CA — When the U.S. Food and Drug Administration convened an advisory panel meeting in September 2015 to discuss the safety and effectiveness of Essure, the permanent birth control device had already been on the market for 13 years.
Thousands of women had a stake in the meeting. They were pushing for panel members to find Essure too unsafe and ineffective to remain on the market, having suffered from life-altering side effects as a result of their own Essure procedures.
These side effects included perforation of the fallopian tubes and uterus, migration of the Essure device, chronic pain, prolonged menstrual bleeding, unintended pregnancies, and the need for hysterectomy, among dozens of others.
Experts on the advisory panel pored over clinical studies involving Essure before making any recommendations to the FDA about the device. These included the premarket clinical studies used to support Essure’s approval and numerous postapproval studies.
Since Essure was approved, it has been the subject of at least six postapproval studies (PAS). These are studies the FDA orders a manufacturer to complete in order to learn more about the safety and effectiveness of devices already on the market.
How can a device that has been the subject of so many mandatory studies cause so many problems in so many women?
We’ll take a closer look at the postapproval studies that helped support the continued use of Essure in the United States. But first we should answer the question, what is Essure?
What is Essure?
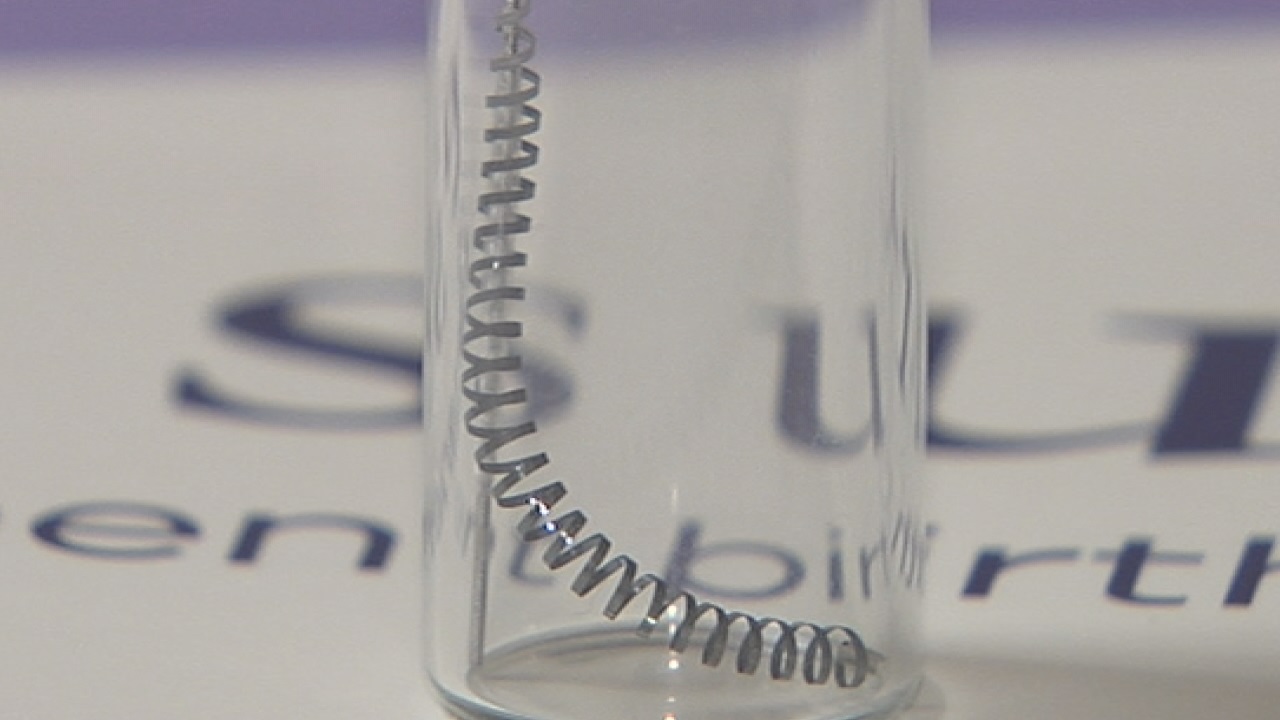
Essure is made of two metal coils that are inserted into the fallopian tubes in the doctor’s office. There is no incision, as the device is inserted through the vagina, and the coils are designed to prevent pregnancy permanently.
Conceptus Inc., then a small medical device startup based near San Francisco, was the original manufacturer of Essure. Pharmaceutical giant Bayer Healthcare purchased Conceptus and the Essure brand in 2013 for $1.1 billion.
The FDA approved Essure in 2002 based on two premarket clinical trials: the Phase II and Pivotal trials. The studies showed the device to be both safe and effective in the vast majority of women who had it implanted and helped speed Essure through the FDA’s premarket approval process on an expedited pathway.
The studies suffered from several weaknesses, however, and as a condition of approval, the FDA required Essure’s manufacturer to conduct two postapproval studies, or PASs.
Since those postapproval studies were ordered, Essure has been the subject of at least four other PASs and a 522 surveillance study.
Essure Postapproval Studies
5 year follow up Study (2002)
Prospective cohort study; no control
 Why was it ordered?
Why was it ordered?
The Phase II and Pivotal premarket trials only followed participants for up to two years. For this reason, the FDA ordered Conceptus to follow these same study participants for another three to gather more long-term data on Essure’s safety and effectiveness.
The study began in 2002 and was concluded in 2008. The results of the study, however, were not published in a peer-reviewed medical journal until September 2015, just ahead of the advisory panel’s meeting.
Limitations
The 5 year follow up PAS included women involved in the Phase II and Pivotal trials — 269 women from Phase II and 657 women from Pivotal. However, only 227 women (84%) from Phase II and 518 from Pivotal (79%) actually underwent the Essure procedure in the original trials.
The women who were enrolled but did not end up getting an Essure procedure were excluded from the data analysis at the end of the trial. This means the safety and effectiveness of Essure was based only on those women who successfully underwent the procedure and does not include those who were excluded or did not pass screening tests.
This could skew the safety and efficacy profile of Essure because it seems not all women can undergo the procedure successfully and this should be reflected in the data.
The data was further limited by inadequate follow-up. Roughly 80% of Phase II participants and less than 90% of Pivotal trial participants were followed-up at three years and those numbers fall to 75% and 82%, respectively, at five years.
Findings
Effectiveness: Both studies observed a 0% pregnancy rate – indicating a 100% effectiveness rate – but that has not been the case in the real world.
Numerous women have become pregnant while using Essure and their risk of dangerous ectopic pregnancies goes up because of it.
The FDA alone received more than 600 reports of pregnancies in women with Essure between 2002 and 2015, at least 96 of which were reported as ectopic.
Safety: During the Phase II trial, 9% of women suffered adverse events after getting Essure. About 5% of those, Conceptus said, were related to period pain, ovulatory pain or changes in menstrual function. The other 4% included perforations, expulsion, unsatisfactory device location and a retained micro-insert fragment.
Perforations accounted for 3% of the adverse events, with seven women suffering from perforations during the trial. One perforation was discovered after the woman had a hysterectomy and another was discovered after the woman had the device removed “due to pain.”
The Pivotal trial appears to have only assessed adverse events that prevented women from relying on Essure for birth control.
Initially, the company reported these types of adverse events affected 21 women (4.5%); however, 9 of those women decided to have Essure inserted a second time. Because those women did not suffer adverse events that prevented them from relying on Essure when it was inserted a second time, they were excluded from the final safety analysis.
Conceptus ultimately reported these types of adverse events in 2.6% of women – thus making Essure’s safety profile even more favorable.
The study can be found on the FDA’s PAS database here.
Newly Trained Physicians study (2002)
Cross-sectional study; historical control
 Why was it ordered?
Why was it ordered?
Conceptus carried out its Newly Trained Physician PAS as a condition of Essure’s approval. The trial documented whether or not doctors newly trained in Essure placement were able to successfully insert the coils on their first attempt.
The study began in 2002 and was completed in 2005. The data was used to create a physician training plan, which the company was compelled to set up by the FDA’s Obstetrics and Gynecology Devices Advisory Panel – the same panel convened in September 2015.
Limitations
When the FDA ordered Conceptus to complete this PAS, the agency specified that it should involve 800 women and 40 physicians.
Conceptus only enrolled 514 women and only 476 (93%) were able to have the device placed. The 38 women who were unable to have Essure placed were excluded from any data analysis.
Conceptus also never explicitly stated whether 40 physicians were involved in the study or not. The study indicated the procedures were performed at 39 different sites, but it does not state how many physicians were involved at each site.
The results of this study were never published in any peer-reviewed journals. There was also no follow-up in this study, implying any adverse events women experienced following the procedure were never collected.
Findings
Effectiveness: During the study, physicians failed to place both Essure coils successfully in 10 of the 476 women who underwent the procedure – a failure rate of about 2%.
Comparatively, 458 of 476 women had both coils placed successfully – a success rate of about 96%.
The reported data leaves 8 women unaccounted for.
Safety: There were 38 malfunctions in 27 cases during the study. In 9 of these, the distal tip of the device was bent, but Conceptus did not consider this to be a malfunction since no adverse events were reported because of the bent tips.
There were 13 adverse events reported during the study, including perforations, pelvic pain, bleeding, light-headedness, increased blood pressure and temporary decreased pulse. These adverse events occurred during the procedure; because there was no follow up, no adverse events that occurred after the procedure were collected.
The study can be found here.
HSG Evaluation Study (2004)
Prospective cohort study; no control
Why was it ordered?
This study was ordered at the same time Conceptus modified a warning statement on the Essure label for physicians in 2004.
HSG (hysterosalpingogram) is the type of procedure used to confirm that the fallopian tubes are fully closed and is usually performed about three months after Essure is placed.
The study began in 2004 and was concluded in 2008; however, the results of the study are not posted on the FDA’s PAS database.
The study can be viewed here.
ESS-305 Study (2007)
Multicenter prospective cohort study; historical control
 Why was it ordered?
Why was it ordered?
The FDA ordered this postapproval study when it approved changes to the design of Essure and the materials used to make it. This included changes to the delivery catheter used to insert the coils into the fallopian tubes.
The study began in 2007 and was terminated early in 2009. It compared newly trained physicians employing the new Essure system with those who had performed more than 25 procedures with the old system.
The results from the 2002 Newly Trained Physicians study were used as a comparator in data analyses.
Limitations
Not all of the study’s results were published in the FDA’s PAS database. The point of the study was to compare the placement rate of newly trained physicians to experienced ones, but the failure and success rates were not reported.
The study was eventually published in the journal Contraception in 2011 – two years after it was completed – and included the failure and success rates not reported on the FDA’s site.
The data was further limited by Conceptus’ failure to include all enrolled study participants in the data analysis. The company enrolled 657 women in the study, but only 584 (89%) of those women had the device successfully placed and were included in data analyses.
Findings
Safety: Conceptus reported only 6 adverse events out of the 584 procedures (1%).
One of the events involved a woman who was hospitalized after the physician punctured her uterus with the hysteroscope (the tool used to look inside the uterus).
Conceptus maintained in the study description that the Essure device did not cause the woman’s injuries.
Effectiveness: Effectiveness data is not available in the FDA’s PAS database.
The study was published in the journal Contraception in 2011 and provides the following results: 37 newly trained physicians and 39 experienced physicians implanted the Essure device in 584 women at 76 different sites around the country. Newly trained physicians had a successful placement rate of 96.1% and experienced physicians had a successful placement rate of 98%.
The study can be viewed here.
OSB Lead-Essure/Post-NovaSure study (2012)
Single-arm, multicenter, prospective, cohort study; no control
Why was it ordered?
The FDA ordered this study when it approved changes to the Essure label that included warnings about the use of the device and the NovaSure procedure.
NovaSure is a type of endometrial ablation procedure in which a doctor surgically removes the lining of the uterus. Women who have unusually heavy or prolonged menstrual periods may seek this type of procedure.
Current Essure labeling indicates women who have had an ablation should tell their doctor as it may affect the Essure procedure. It also indicates that the ablation and Essure procedure should not be performed on the same day.
This study began in 2012 and is expected to be completed by February 2017.
Limitations
This study, like all Essure trials conducted by its manufacturer, lacks a control. Controls are important in clinical trials because they help eliminate alternative explanations and increase the reliability of the results. Controls are considered one element of the “gold standard” of clinical trials.
Because this study is not yet complete, it is difficult to determine whether or not other limitations exist without the full trial being published.
Findings
This is an ongoing study involving a minimum of 220 women between the ages of 21 and 50 who already had Essure and are seeking treatment of unusually heavy or prolonged periods (NovaSure).
The study will look at how effective Essure is at preventing pregnancy in women who have the NovaSure procedure. Pregnancy data will be collected at 1 year and 3 years post-NovaSure procedure.
The study’s five-year final report is expected by Feb. 22, 2017.
The study can be found here.
ODE Lead-Post Approval Study (2015)
Prospective, multi-center, international study; no control
 Why was it ordered?
Why was it ordered?
This study was ordered by the FDA to support changes made to the Essure label in 2015. The new label updated the approved tests used to confirm the correct placement of the Essure coils.
Since Essure was approved, physicians have used the HSG test to confirm correct placement of the device. HSGs use contrast dye and x-rays to make sure the tubes are closed.
The test is typically performed three months after insertion and women must use an alternative form of birth control (i.e. birth control pill, condoms) until the confirmation test shows the tubes are indeed closed.
The updated label indicated physicians could now use a transvaginal ultrasound as first-line confirmation at three months after insertion, along with the HSG test. In order to support this change, the FDA ordered this 10-year follow-up PAS to look at how many pregnancies occur using this new confirmation testing method.
Limitations
Since this study is ongoing, it is difficult to see what sort of limitations it might suffer from. On its face, the study’s main limitation is that it lacks a control — an important aspect of clinical trials that help reduce uncertainty about the study’s results.
Findings
This ongoing study is a continuation of a premarket study and is intended to follow women up to 10 years following insertion of Essure.
The study’s main outcome is to see how many pregnancies occur within the first year when physicians use the vaginal ultrasound/HSG confirmation method. The study also aims to evaluate the number of confirmed pregnancies at 10 years.
620 women were reportedly enrolled in the trial. Bayer submitted the one-year report on time in July 2016. It is slated to submit a report each year until the ten-year mark in 2025.
Essure 522 Study
Device manufacturers must complete postapproval studies or the FDA could take action against the companies, including ordering more studies be conducted.
These additional studies are called postmarket surveillance studies, or 522 studies. The FDA ordered the manufacturer of Essure to conduct a 522 study after the advisory panel convened in September 2015.
The study protocol was approved by the FDA in September 2016.
 Postmarket Surveillance Study
Postmarket Surveillance Study
Open-label, non-randomized, prospective observational cohort study; concurrent control
Why was it ordered?
Following the September 2015 advisory meeting, the FDA ordered Bayer to conduct this postmarket surveillance, or 522, study.
The main goal of the study is to compare the safety and effectiveness of Essure compared to traditional tubal ligation (getting one’s tubes tied).
The agency states on its website that more clinical data was needed “to better define and understand certain outcomes and events that may be associated with Essure when compared to women who undergo tubal ligation.”
The study will compare side effects experienced by women who get Essure compared with women who get their tubes tied and the results will be used by the FDA in any future action. The six-year study is scheduled to run through September 2023.
Limitations
This study is in its early phases, but one limitation it currently suffers from is non-randomization.
Randomization in trials is important because it minimizes selection bias and helps ensure the sample is representative of the larger population from which it is drawn. Study results may not be reliable if there are important differences between the study cohort and the population at large. (Source: National Cancer Institute)
Findings
This is an ongoing study intended to enroll 2,800 women between the ages of 21 and 45 who will undergo either Essure or tubal ligation (1,400 per group).
The main endpoints of the study include:
- Chronic lower abdominal and/or pelvic pain
- Abnormal uterine bleeding (new onset or worsening)
- Hypersensitivity and allergic reactions, and autoimmune disorders (new onset) or autoimmune- like reactions
- Invasive gynecologic surgery including Essure insert removal
- Pregnancy
- Other adverse events
The study can be viewed here.
Essure Lawsuits
Women harmed by Essure began filing lawsuits against Essure’s current manufacturer Bayer Healthcare last year and those lawsuits have begun gaining traction in both state and federal courts throughout the country.
Several judges have ruled certain claims could proceed in court despite the federal preemption granted to Essure through the FDA’s premarket approval process.
If you or a loved one were injured as a result of Essure, you may be entitled to financial compensation for lost wages and medical costs.
Call the National Injury Attorneys, LLC today to speak with a member of our legal team. We can help answer your questions and see if you qualify for an Essure Lawsuit with our free case evaluation. Call today at 1-800-214-1010 or use the form on the right-hand side of your screen.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- When Pet Owners Fail to Control Aggressive Dogs: Legal Options for Bite Victims in California
- Rideshare Driver or Passenger? Legal Steps to Take After a California Uber or Lyft Accident
- Crosswalk Accidents: When California Drivers Fail to Yield and Pedestrians Pay the Price
- Parents, Teens, and Texting: Addressing the Dangers of Distracted Driving Among Young Drivers in California
- California Rideshare Accidents Involving Minors: What Parents Need to Understand






