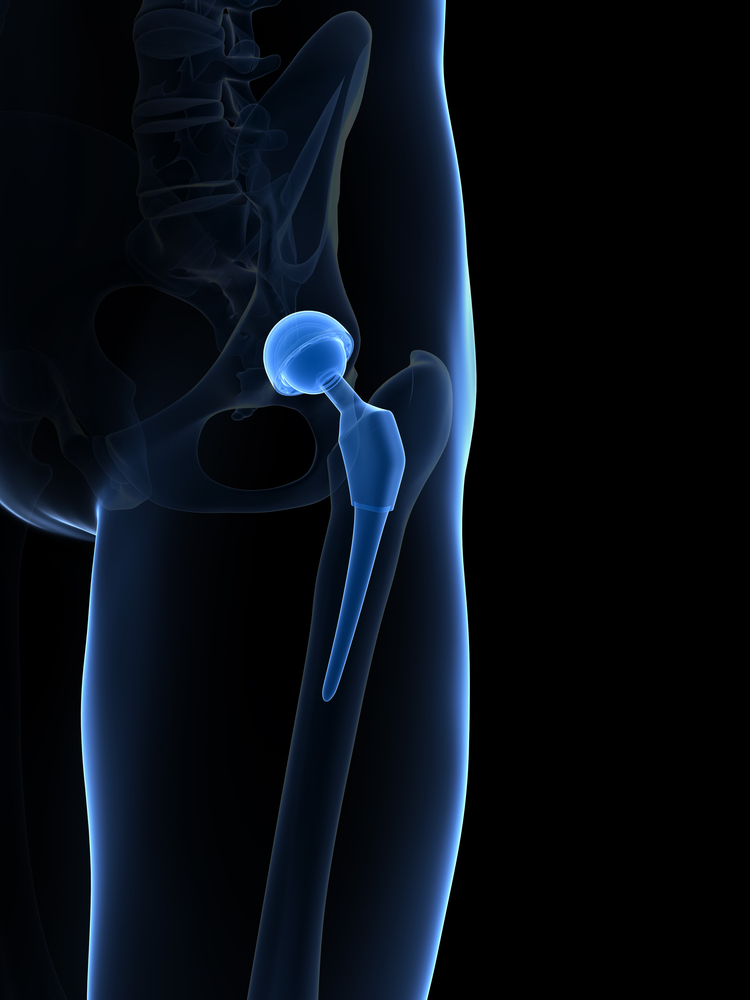
On October 2nd, 2015, MicroPort Orthopedics issued a Class I recall for thousands of hip implant parts that can potentially fracture and lead to serious complications. The recall includes 10,825 modular-neck components known as the Profemur Neck Varus/Valgus CoCR Part 1254, made from June 15, 2009 to July 22, 2015.
What is the MicroPort Profemur Hip Implant?
Formerly manufactured by a division of Wright Medical, Profemur products have been made and sold by Chinese orthopedic manufacturer MicroPort ever since the company acquired Wright Medical back in January 2014. The Profemur modular-neck is a component between the femoral stem and femoral head, which is part of a variety of hip replacement systems that use different parts to help surgeons fit the implant to match a patient’s unique anatomy. While these implants are described as “streamlined” and “easier to insert,” by MicroPort, they are prone to fracture.
MicroPort Profemur FDA Recall
MicroPort began the recall on August 7th after receiving several reports of an “unexpected rate of fractures after surgery.” A broken hip implant can potentially cause sudden, intense pain and limited mobility. The company warns patients in their recall:
“An acute fracture will require revision surgery to remove and replace the neck and stem components. Acute fracture and emergency revision surgery is a serious adverse health consequence and could lead to neurovascular damage, hematoma, hemorrhage, and even death.”
A Class I recall is the most serious form of recall the FDA can issue and patients should seek emergency medical attention if they develop any of these symptoms below:
- Sudden and severe pain in the hip joint
- Problems walking
- Falling or other severe trauma to the hip or leg
- Tingling or loss of feeling in the leg (numbness)
The Class I Recall is described as:
“[T]he most serious type of recall and involve situations in which there is a reasonable probability that use of these products will cause serious adverse health consequences or death.”
Injured MicroPort Profemur Patients Compensation
Manufacturers have a duty to provide healthy, safe products to their patients. If there are serious risks linked with their devices, they should be required to provide proper warnings as well. If a company fails to properly warn patients of these risks, they could be held liable in lawsuits for any injuries that result.
If a patient dies due to complications from a MicroPort Profemur, family members could be entitled to compensation for the wrongful death of their loved one, including:
- Conscious pain and suffering of a loved one prior to death
- Pain, suffering and mental anguish from the loss of a loved one
- Funeral expenses
Note: The information provided in this article is based on reports from publicly available sources, including news outlets, police reports, and eyewitness accounts. National Injury Help has not independently verified all details of the reported incident. If you find any inaccurate or outdated information, please contact us, and we will review and update the content as appropriate. The photo used in this post is for illustrative purposes only and does not depict the actual scene of the incident.
Disclaimer: The content of this article is intended for informational purposes only and does not constitute legal advice or establish an attorney-client relationship with National Injury Help. For legal assistance specific to your case, we encourage you to contact a qualified attorney.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent posts
- Phoenix, AZ – One Dead, One Injured in Two-Vehicle Crash at 35th Ave & Northern Ave
- Tucson, AZ – Injuries Reported in Crash at Stone Ave & Roger Rd
- Tucson, AZ – Reportan heridos en un choque en Stone Ave y Roger Rd
- Tucson, AZ – Injuries Reported in Accident at Grant Rd & Beverly Ave
- Phoenix, AZ – Un muerto y un herido en choque de dos vehículos en 35th Ave y Northern Ave