Did a defective device used in trials lead to Xarelto being approved by the FDA?
December 9, 2015 — San Diego,CA Xarelto, a blood thinning drug, has come under scrutiny by both European and U.S. drug regulators for a possible defective blood clot testing instrument used to measure the rate of clots forming. The tests were performed between Xarelto and much older drug called warfarin.
The question is did Bayer, the manufacturer of Xarelto known as rivaroxaban, know that the testing devices were possibly in error? If so this could present some problems for the company.
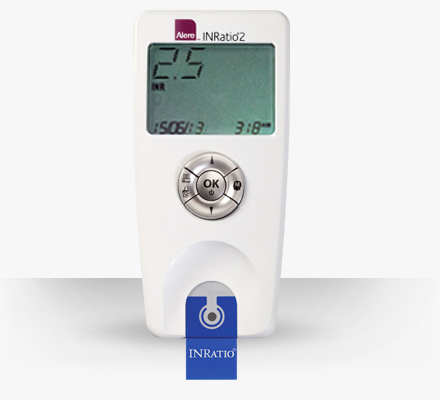
The device in question is called an INR and measures how fast blood begins to clot. The Alere INRatio and INRatio2 PT/NR Monitors were recalled in December 2014 after delivering false low test results.
The FDA is aware of the investigation and will monitor the story further.
When this investigation news broke shares of Bayer’s stock dropped by 1.7%, but analysts think the impact would be nominal.
Xarelto is Bayer’s best selling drug with revenue in 2014 of $1.8 billion, and $1.6 billion in the first nine months of 2015.
Xarelto is an anticoagulant that is used for people who have atrial fibrillation (irregular heartbeat) or to prevent DVT (Deep Vein Thrombosis) and other risk of blood clots associated with hip or knee replacement surgery.
Kepler Cheuvreux’s Fabian Wenner issued this statement: “Given that … probably hundreds of different devices were used to assess the control group patients, a single defective device is unlikely to have a statistically relevant effect on the overall outcome.”
We at National Injury Attorneys, LLC will continue to watch this story develop and will hold Bayer accountable for any misrepresentation of clinical testing.
For those that have been prescribed Xarelto, talk to your doctor before stopping this drug.
If you or someone you know has had an unstoppable bleeding event from this drug, contact us today for a free review of the situation. Click the banner below to start the claims evaluation.
Note: The information provided in this article is based on reports from publicly available sources, including news outlets, police reports, and eyewitness accounts. National Injury Help has not independently verified all details of the reported incident. If you find any inaccurate or outdated information, please contact us, and we will review and update the content as appropriate. The photo used in this post is for illustrative purposes only and does not depict the actual scene of the incident.
Disclaimer: The content of this article is intended for informational purposes only and does not constitute legal advice or establish an attorney-client relationship with National Injury Help. For legal assistance specific to your case, we encourage you to contact a qualified attorney.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- Tucson, AZ – Injury-Causing Crash at Broadway St and Kolb Blvd
- Tucson, AZ – Crash With Injuries at Wetmore Rd & Flowing Wells Rd
- Phoenix, AZ – Chain-Reaction Crash on Cave Creek Rd
- The One-Bite Myth in California: Why Dog Owners Can’t Claim ‘They Didn’t Know’
- Tolleson, AZ – Accidente con heridos entre bicicleta y auto en 99th Ave