New 2016 Law May add Pressure on Essure Birth Control Device
January 6, 2016 — San Diego, CA The new law that just went into effect last week is titled the “Microbead-Free Waters Act of 2015.”
This brand new Act of Congress stops “manufacture and introduction into interstate commerce of rinse-off cosmetics containing intentionally-added plastic microbeads.” This is a win for the environment and it may also prompt a deeper look at similar types of products, one being PET fibers.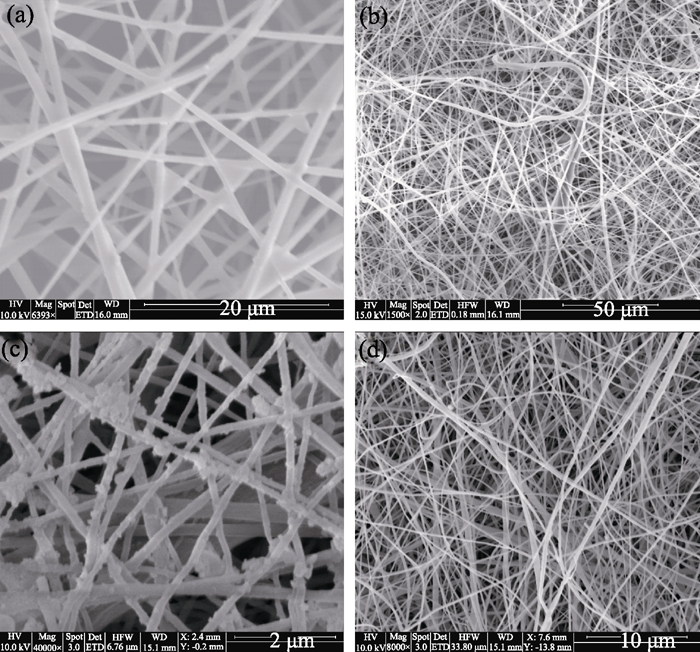
Bayer’s Essure product contains polyethylene terephthalate (PET) fibers. It’s well known that PET fibers are dangerous to our bodies, but it was still approved by the F.D.A.
One manufacturer of PET has published a Material Safety Data Sheet (MSDS) that warns against the use of its products in medical procedures that require implants in the body.
It clearly states: “CAUTION: Do not use in medical applications involving permanent implantation in the human body”
Some scientists including Dr. Margaret Aranda, are concerned about the use or PET fibers in the Essure device since it is known that they degrade from heat and time.
In an article on the subject she writes:
“The by-products of PET degradation, are acetyladehyde, a toxic intermediate in the metabolism of alcohol responsible for much of the liver disease in chronic alcoholics and antimony, a semi-metallic chemical element that is deleterious to health. Acetyladehyde is pervasive in airborne environments as a by-product wood smoke and other thermal reactions. Whether by inhalation or ingestion, acetyladehyde is a carcinogen. Should pregnancy occur in the presence of acetylaldehyde exposure, it does cross the placenta and induce skeletal malformations, reduced birth weight, and increased postnatal mortality. Given its placement in the Fallopian tubes, follicular acetylaldehyde exposure could be predicted to induce changes in follicular morphology and perhaps even germ cell health for subsequent generations. Acetyladehyde is not something one wants leaching into the fallopian tubes, particularly when device placement is incorrect and pregnancy prevention nowhere near 100%.”
It’s possible that this could be the source of so many women having terrible side effects from the Essure device. There haven’t been any studies done yet to correlate this theory, but when a manufacturer states that it’s products shouldn’t be used in the body…leaves one to wonder.
Note: The information provided in this article is based on reports from publicly available sources, including news outlets, police reports, and eyewitness accounts. National Injury Help has not independently verified all details of the reported incident. If you find any inaccurate or outdated information, please contact us, and we will review and update the content as appropriate. The photo used in this post is for illustrative purposes only and does not depict the actual scene of the incident.
Disclaimer: The content of this article is intended for informational purposes only and does not constitute legal advice or establish an attorney-client relationship with National Injury Help. For legal assistance specific to your case, we encourage you to contact a qualified attorney.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent post
- Tucson, AZ – Injury-Causing Crash at Broadway St and Kolb Blvd
- Tucson, AZ – Crash With Injuries at Wetmore Rd & Flowing Wells Rd
- Phoenix, AZ – Chain-Reaction Crash on Cave Creek Rd
- The One-Bite Myth in California: Why Dog Owners Can’t Claim ‘They Didn’t Know’
- Tolleson, AZ – Accidente con heridos entre bicicleta y auto en 99th Ave




