Just months after the manufacturer C.R. Bard agreed to pay a $200 million settlement over its vaginal mesh, the company is now facing more legal issues over their defective device, the IVC filter. The company’s behavior has crossed the line from mere negligence into criminal fraud.
IVC Filter Complications
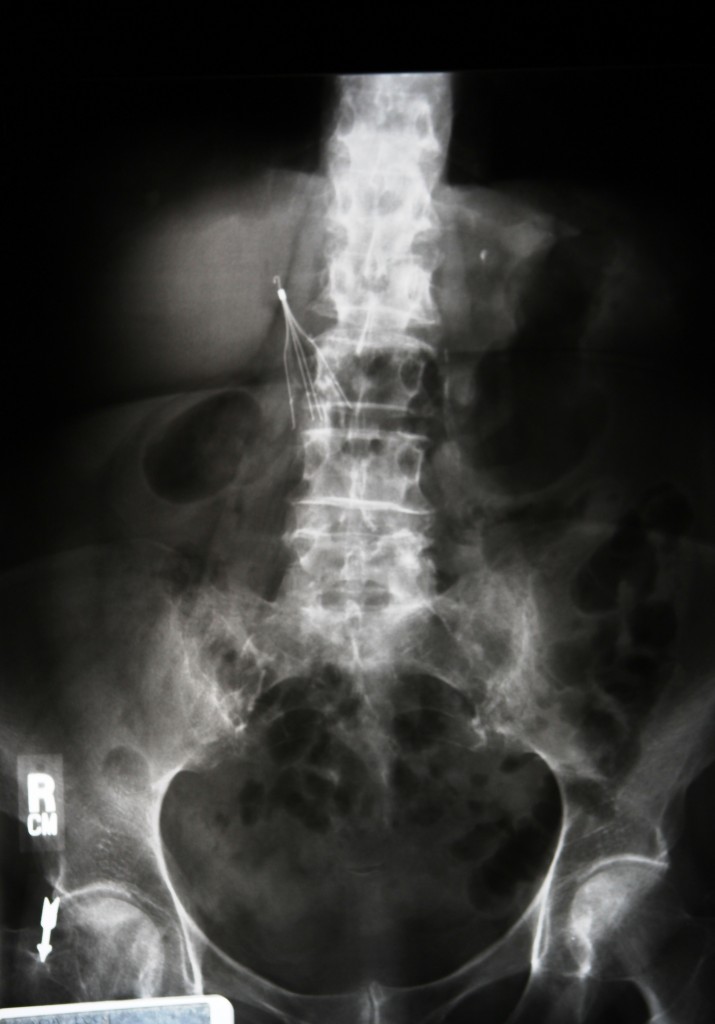
The IVC, or inferior vena cava, filter resembles a common cellar spider and is designed for implantation into the major vein that carries deoxygenated blood from the lower body into the right atrium, or collection chamber, of the heart. The purpose of the device is to prevent pulmonary embolism, or blockage of the flow of blood from a clot. Despite serious concerns about the IVC’s safety and effectiveness, as well as numerous recommendations that they be used only for high-risk cases, close to a quarter-million such devices are implanted in patients each year.
In the news recently, NBC News investigated a report dated December 15, 2004 from Dr. John Lehmann, a doctor hired by C.R. Bard to conduct an independent study of the IVC filter. Dr. Lehmann raised dangerous concerns about the device and found that it was more risky than any similar filter on the market. Lehmann urged that “further investigation of the filter in relation to migration and fracture is urgently warranted.”
Over the next six years, the FDA received more than 900 reports of adverse events linked to the Recovery IVC filter, including fracture and migration. Small, metal parts can become wedged in veins, leading to perforation and death. Close to 30 fatalities have been attributed to Bard’s IVC filter.
Bard’s Criminal Conduct
In light of all of Dr. Lehmann’s reports and warnings, why did Bard continue with the manufacturing and sale of the IVC filter? Unfortunately, the reason is all too common with big pharmaceutical companies: profit. Executives with Bard realized that a redesign was in order, however the new model wouldn’t be ready for at least a year. Instead of pulling the earlier model, Bard made the decision to not submit the required report to the FDA, which put the company in direct violation of federal law.
The story gets worse. When a regulatory specialist for Bard, Katy Fuller, refused to sign off on an FDA application for approval of the filter, the company decided to forge her signature. You can read more about this in our blog post.
Since the new model was released in 2005, which isn’t much better than the original, there have been over 900 adverse events reported. In 2010, a study published in the Archives of Internal Medicine revealed that 16% of the IVC filters implanted in patients had at least one fractured strut. The researchers concluded that “The Bard Recovery and Bard G2 filters had high prevalence of fracture and embolization, with potentially life-threatening sequelae [consequences].”
The initial response of Bard was to hire publicists to spin the company’s image. Since plaintiffs started coming forward with lawsuits, Bard has filed numerous motions to have the actions dismissed. This past summer, the FDA sent a warning letter to the company, citing several violations of federal law. including the sale of “adulterated” and “misbranded” products, failure to report adverse events and withholding safety information from consumers.
Contact our IVC filter lawyers and attorneys today – you may receive a large cash settlements from claims filed.
Note: The information provided in this article is based on reports from publicly available sources, including news outlets, police reports, and eyewitness accounts. National Injury Help has not independently verified all details of the reported incident. If you find any inaccurate or outdated information, please contact us, and we will review and update the content as appropriate. The photo used in this post is for illustrative purposes only and does not depict the actual scene of the incident.
Disclaimer: The content of this article is intended for informational purposes only and does not constitute legal advice or establish an attorney-client relationship with National Injury Help. For legal assistance specific to your case, we encourage you to contact a qualified attorney.
Free Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Contact Us today for a FREE, Immediate Case Evaluation
Categories
Recent posts
- Tempe, AZ – Injuries Reported in Crash at Baseline Rd & Beck Ave
- Litchfield Park, AZ – UPDATE: Man Arrested in Fatal Crash at Camelback Rd & Litchfield Rd
- Litchfield Park, AZ – ACTUALIZACIÓN: Hombre arrestado tras choque fatal en Camelback Rd & Litchfield Rd
- Tucson, AZ – Choque deja heridos en Bilby Rd & Morris St
- Tempe, AZ – Heridos en choque en Baseline Rd & Beck Ave